Method for producing magnesium oxide with co-production of ammonium chloride by taking magnesium carbonate hydrate as intermediate
A technology of magnesium carbonate and hydrate, applied in the directions of magnesium oxide, magnesium carbonate, ammonium halide, etc., can solve the problems of high cost and large amount of ammonium bicarbonate in production and consumption, and achieve the effect of increasing consumption and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
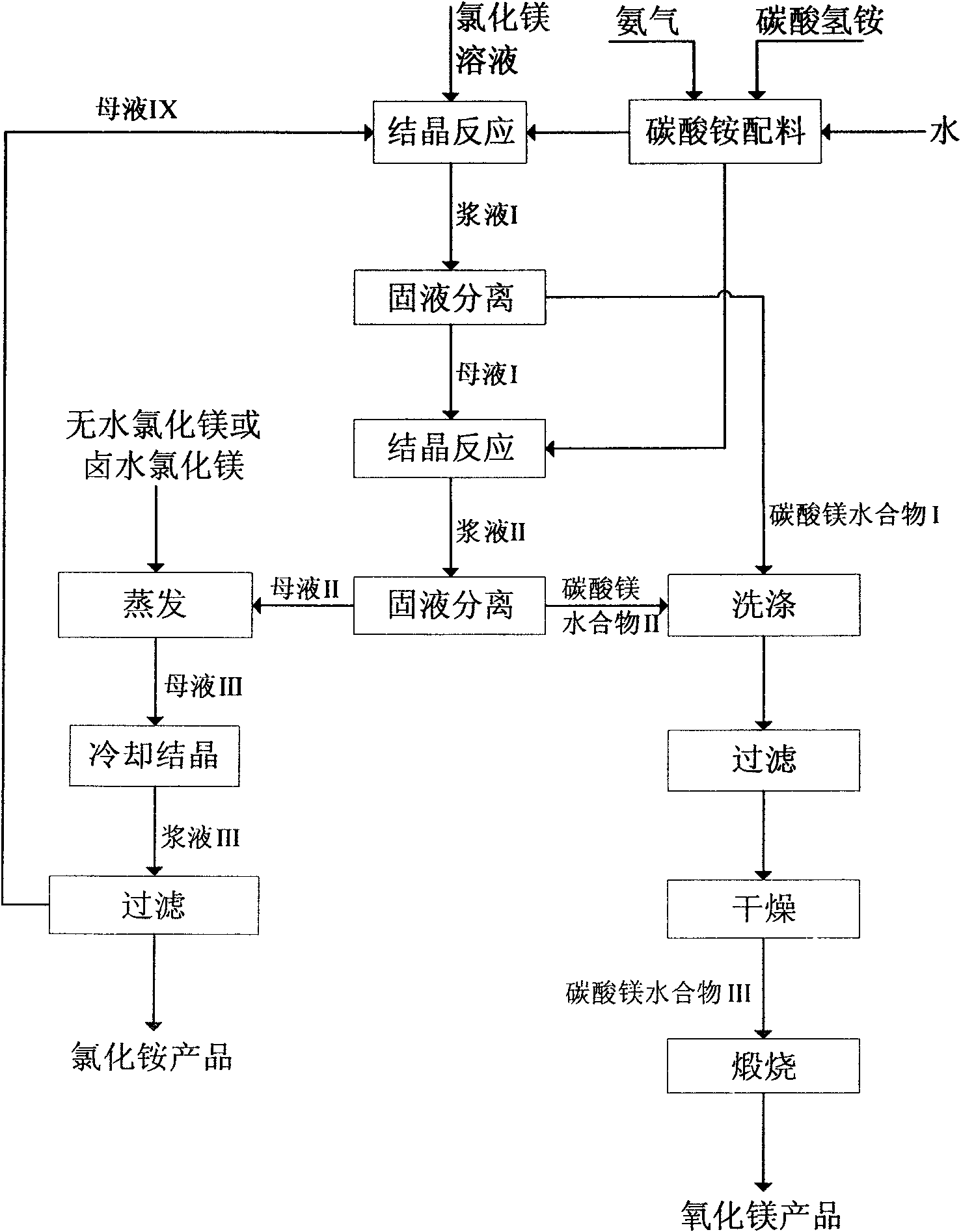
[0069] See figure 1 .
[0070] 1) Prepare 2000ml of magnesium chloride solution with water using the anhydrous magnesium chloride of sponge titanium by-product, wherein the concentration of magnesium chloride is 3mol / L; prepare 2000ml of ammonium bicarbonate solution with water, and the concentration of ammonium bicarbonate is 3mol / L; pass into the ammonium bicarbonate solution Ammonia, the molar ratio of ammonium bicarbonate to ammonia is 2:1.
[0071] 2) The magnesium chloride solution prepared in step 1) is passed into the crystallization reactor at a certain flow rate, and the ammonium bicarbonate solution after absorbing ammonia gas is passed into the magnesium chloride solution, and the reaction crystallization is obtained after reaction crystallization at a reaction temperature of 20°C for 1 hour. Serum I. The volume of the ammonium bicarbonate solution introduced after absorbing ammonia is 0.6 times the volume of the magnesium chloride solution, and the mol ratio of ...
Embodiment 2
[0076] See figure 1 .
[0077] 1) Use seawater brine magnesium chloride to prepare 2000ml of magnesium chloride solution with pure water, wherein the concentration of magnesium chloride is 5mol / L; prepare 2000ml of ammonium bicarbonate solution with pure water, and the concentration of ammonium bicarbonate is 5mol / L; feed ammonia into the ammonium bicarbonate solution Gas, the molar ratio of ammonium bicarbonate to ammonia is 2:1.
[0078] 2) The magnesium chloride solution prepared in step 1) is passed into the crystallization reactor at a certain flow rate, and the ammonium bicarbonate solution after absorbing ammonia gas is passed into the magnesium chloride solution, and the reaction temperature is 90 ° C. After crystallization for 6 hours, the obtained Serum I. The volume of the ammonium bicarbonate solution after absorbing the ammonia that is introduced is 0.4 times of the magnesium chloride solution volume, and the mol ratio of ammonium bicarbonate and magnesium chlor...
Embodiment 3
[0083] See figure 1 .
[0084] 1) Use salt lake brine to add pure water to prepare 2000ml of magnesium chloride solution, wherein the concentration of magnesium chloride is 4mol / L; prepare 2000ml of ammonium bicarbonate solution with pure water, and the concentration of ammonium bicarbonate is 4mol / L; feed ammonia into the ammonium bicarbonate solution , the molar ratio of ammonium bicarbonate to ammonia is 2:1.
[0085] 2) The magnesium chloride solution prepared in step 1) is passed into the crystallization reactor at a certain flow rate, and the ammonium bicarbonate solution after absorbing ammonia gas is passed into the magnesium chloride solution, and the reaction temperature is 80° C. After crystallization for 4 hours, the obtained Slurry I; The volume of the ammonium bicarbonate solution after absorbing the ammonia that passes through is 0.5 times of the magnesium chloride solution volume, and the molar concentration ratio of ammonium bicarbonate and magnesium chloride...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com