Magnetic nanocomposite fiber and preparation method and application thereof
A composite fiber and magnetic nano technology, applied in the preparation of organic compounds, carboxylate preparation, chemical instruments and methods, etc., to achieve the effect of simple process, short cycle and cheap price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
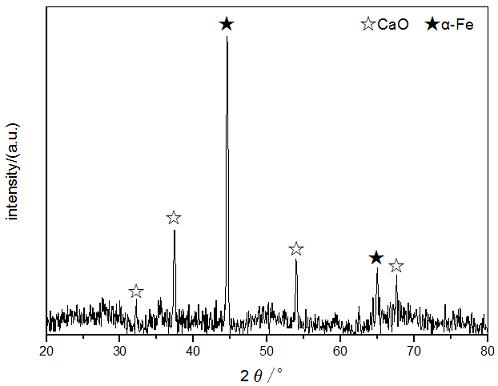
[0026] Example 1 ( CaO / α-Fe Fiber M(CaO):M (α-Fe)=1:1 ):
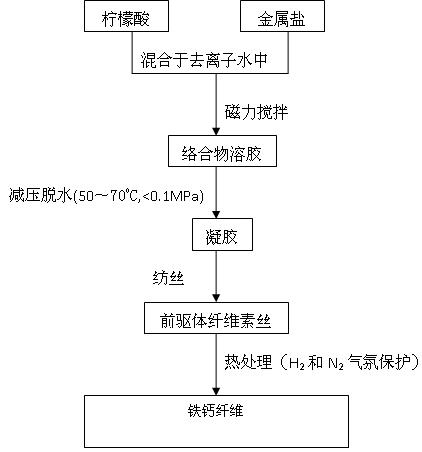
[0027] Step 1: get 5.618g calcium nitrate (Ca(NO 3 ) 2 4H 2 O), 9.612g ferric nitrate (Fe(NO 3 ) 3 .9H 2 O), 15g citric acid (CA:C 6 h 8 o 7 ·H 2 O), mixed in 150ml deionized water, Ca 2+ The molar concentration of Fe is 0.159mol / L, Fe 3+ The molar concentration is 0.159mol / L, and the raw material molar ratio is: CA: Fe 3+ : Ca 2+ =3:1:1, then carry out magnetic stirring for 20 hours;
[0028] Step 2: Then put the precursor solution into a vacuum rotary evaporator, depressurize at 60°C, the pressure is less than 0.1Mpa, dehydrate for about 30 minutes, and obtain a gel-like colloidal substance;
[0029] Step 3: Put the gel obtained in step 2 into an oven, dry and dehydrate at 60°C, and place it in the oven for about 1 hour, then pull the gel into gel cellulose filaments, and the cellulose filaments Dry in a crucible at 100°C.
[0030] Step 4: Put the fiber precursor in H 2 , N 2 In the atmosphere...
Embodiment 2
[0031] Example 2 ( CaO / α-Fe Fiber M(CaO):M (α-Fe)=1:1.5 ):
[0032] Step 1: get 4.214g calcium nitrate (Ca(NO 3 ) 2 4H 2 O), 10.814g ferric nitrate (Fe(NO 3 ) 3 9H 2 O), 15g citric acid (CA:C 6 h 8 o 7 ·H 2 O), mixed in 150ml deionized water, Ca 2+ The molar concentration of Fe is 0.119mol / L, Fe 3+ The molar concentration is 0.178mol / L, and the raw material molar ratio is: CA: Fe 3+ : Ca 2+ =3:1.5:1, then carry out magnetic stirring for 20 hours;
[0033] Step 2: Then put the precursor solution into a vacuum rotary evaporator, depressurize at 60°C, the pressure is less than 0.1Mpa, dehydrate for about 30 minutes, and obtain a gel-like colloidal substance;
[0034]Step 3: Put the gel obtained in step 2 into an oven, dry and dehydrate at 60°C, and place it in the oven for about 1 hour, then pull the gel into gel cellulose filaments, and the cellulose filaments Place in a crucible and dry at 100°C;
[0035] Step 4: Put the fiber precursor in H 2 , N 2 In th...
Embodiment 3
[0036] Example 3 ( CaO / α-Fe Fiber M(CaO):M (α-Fe)=1:2 ):
[0037] Step 1: get 3.746g calcium nitrate (Ca(NO 3 ) 2 4H 2 O), 12.817g ferric nitrate (Fe(NO 3 ) 3 .9H 2 O), 15g citric acid (CA:C 6 h 8 o 7 ·H 2 O), mixed in 150ml deionized water, Ca 2+ The molar concentration of Fe is 0.106mol / L, Fe 3+ The molar concentration is 0.212mol / L, and the raw material molar ratio is: CA: Fe 3+ : Ca 2+ =4.5:2:1, then carry out magnetic stirring for 20 hours;
[0038] Step 2: Then put the precursor solution into a vacuum rotary evaporator, depressurize at 60°C, the pressure is less than 0.1Mpa, dehydrate for about 30 minutes, and obtain a gel-like colloidal substance;
[0039] Step 3: Put the gel obtained in step 2 into an oven, dry and dehydrate at 60°C, and place it in the oven for about 1 hour, then pull the gel into gel cellulose filaments, and the cellulose filaments Place in a crucible and dry at 100°C;
[0040] Step 4: Put the fiber precursor in H 2 , N 2 In t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com