Liposome medicament containing cholesterol PEG modifier and preparation method thereof
A liposome and cholesterol technology is applied in the field of preparing medicines for treating tumor diseases, and can solve the problems of hindering the loading of vinorelbine, poor drug retention capacity, and reducing drug loading efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1 liposome preparation method
[0014] Mix phospholipids, such as hydrogenated soybean lecithin (or bispalmitate lecithin or bis myristate lecithin), cholesterol in a weight ratio of 3:1, and an appropriate amount of PEG-modified cholesterol, and dissolve in 95% tert-butyl In alcohol, the organic solvent was removed by lyophilization to obtain a loose lipid powder, which was then hydrated with an internal phase solution containing counterions to form blank liposomes. Use high-pressure extrusion equipment or micro-fluidic equipment to reduce the particle size of blank liposomes, and then use column chromatography or dialysis to replace the counterions outside the liposome with sucrose / histidine solution, so that an ion gradient is formed inside and outside the lipid membrane. The drug aqueous solution and the liposome suspension are mixed and incubated to obtain.
Embodiment 2
[0015] Example 2 Comparison of triethylamine sulfosalicylate internal phase, different long-circulation materials, and different drug lipid ratios of vinorelbine drug-loaded encapsulation efficiency
[0016] Internal phase: 300mM triethylamine sulfosalicylate solution
[0017] Lipid phase: Content of mPEG2000-DSPE or cholesterol-PEG: 8.3%.
[0018] The preparation method is the same as in Example 1.
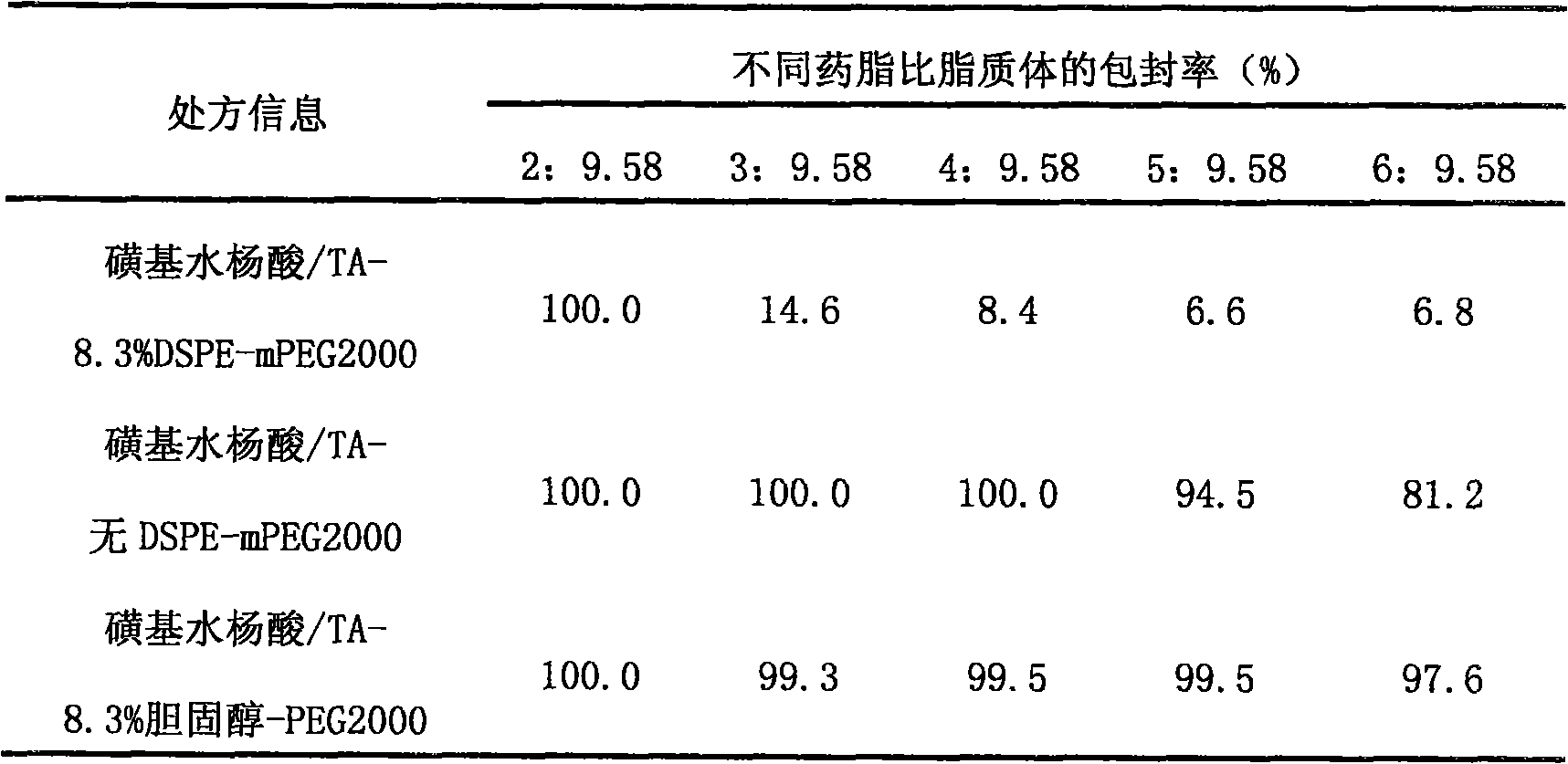
[0019] Table 1 Different lipid-drug ratios, encapsulation efficiency of different long-circulation materials vinorelbine liposomes
[0020]
[0021] Conclusion: When the prescription contains 8.3% DSPE-mPEG2000, even if triethylamine sulfosalicylate, which has a strong drug retention ability, is used as the internal phase, when the drug-lipid ratio reaches 3:9.58 or above, the encapsulation rate also gradually decreased. In the absence of DSPE-mPEG2000, a high encapsulation efficiency can be maintained when the drug-lipid ratio reaches 6:9.58. This phenomenon indicated tha...
Embodiment 3
[0022] Example 3 Comparison of triethylamine sulfosalicylate internal phase, different long-cycle materials, and different ratios of drug lipids to vincristine drug loading and encapsulation
[0023] Internal phase: 300mM triethylamine sulfosalicylate solution
[0024] Lipid phase: Content of mPEG2000-DSPE or cholesterol-PEG: 8.3%.
[0025] The preparation method is the same as in Example 1.
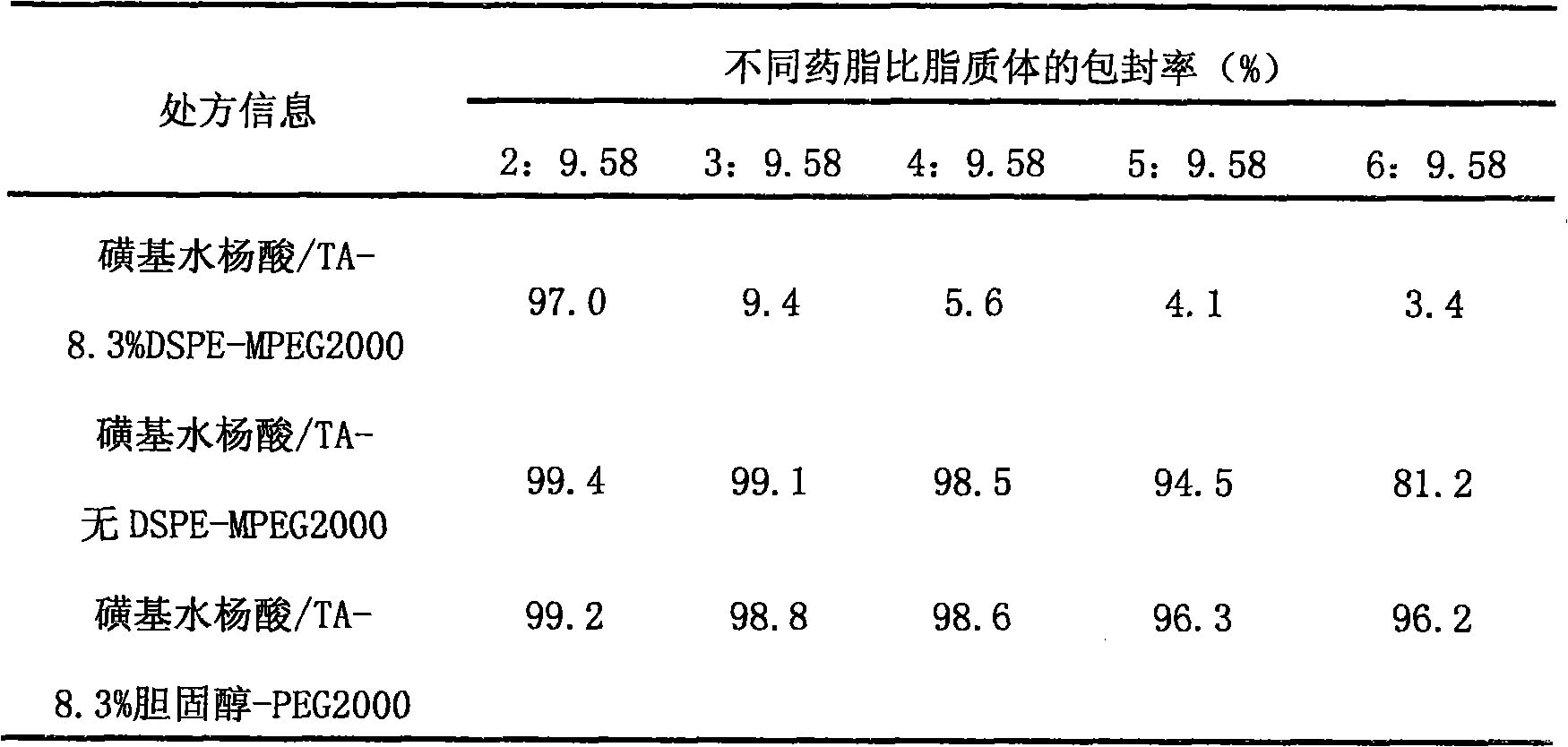
[0026] Table 2 Encapsulation efficiency of different drug-lipid ratios with different long-circulating phospholipid vincristine liposomes
[0027]
[0028] Conclusion: The presence of 8.3% DSPE-MPEG will affect the loading of vincristine liposomes in the internal phase of triethylamine sulfosalicylic acid salt, and the effect will be more significant when the drug-to-lipid ratio increases, and the drug can hardly be loaded. However, after replacing DSPE-mPEG2000 with cholesterol-PEG2000, the drug-lipid ratio was increased to 6:9.58, and the drug was still well loaded, and the encaps...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com