Pesticide composition
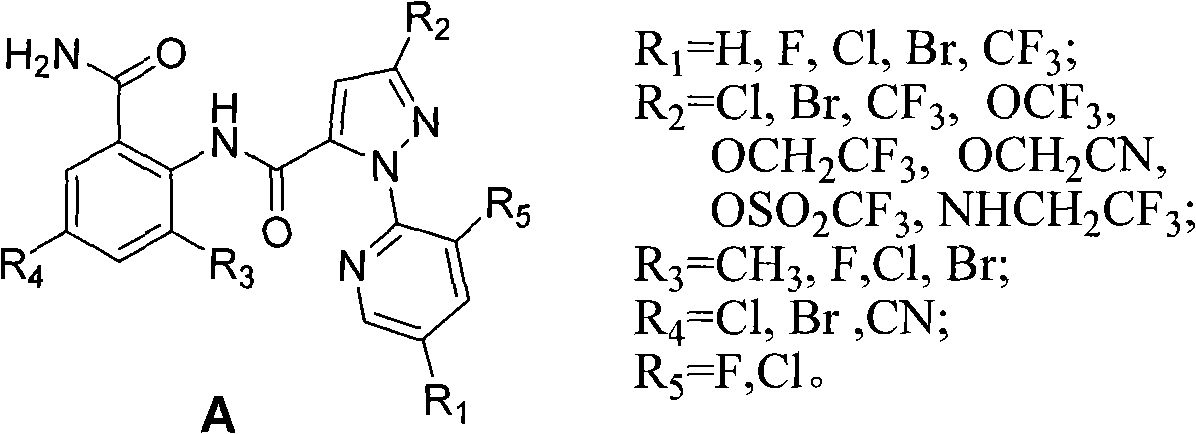
A technology of composition and insecticide, applied in the direction of insecticide, acaricide, biocide, etc., can solve the problem of in-depth research and development of the compound of general formula A without any reports, so as to delay the resistance of pests and reduce the use of insecticides. Cost, effect of reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
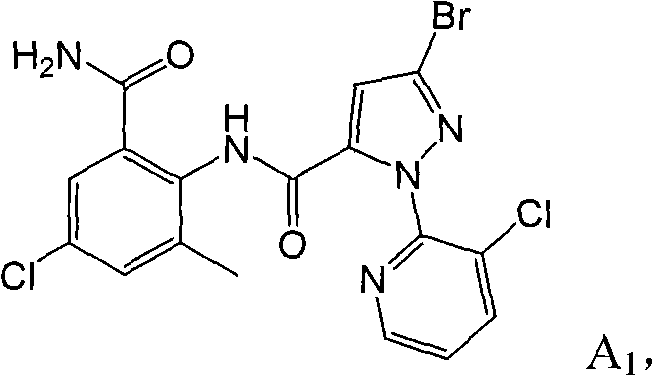
[0053] The first active component compound A1 in the composition can be prepared by the following three methods.
[0054] Synthetic method 1
[0055]
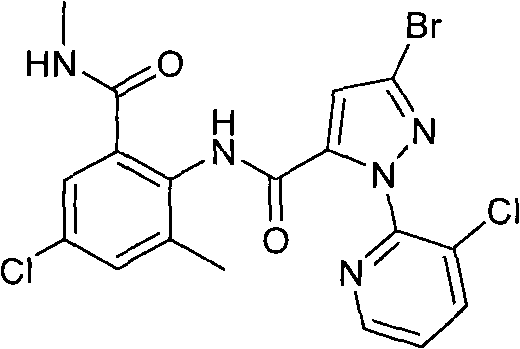
[0056] Take the intermediate 2-amino-5-chloro-3-methylbenzamide (0.39g, 2.0mmol) (refer to patent WO2006062978 for synthesis) in a reaction flask containing 10ml of tetrahydrofuran, and slowly add 3-bromo-1- (3-Chloropyridin-2-yl)-1H-pyrazole-5-formyl chloride (0.70g, 2.2mmol), add 1ml triethylamine dropwise under stirring, stir at room temperature overnight, TLC detects that the reaction is complete, and removes under reduced pressure The solvent was extracted with ethyl acetate and water, the organic layer was washed twice with saturated sodium carbonate, and dried to obtain 0.83 g (1.77 mmol) of a light yellow solid with a yield of 88.5%. The melting point is 236-237°C.
[0057] 1 HNMR (300MHz, CDCl 3 )δ10.30(s, 1H, Ph-NH), 8.47(dd, 1H, Pyridin-6-H), 8.10(dd, 1H, Pyridin-4-H), 7.72(s, 1H, Ph-3- H), 7.56(dd, 1H, Pyrid...
preparation example
[0066] The percentages in the proportioning ratio of all the composition preparations are percentages by weight.
[0067] Preparation of 10% EC
[0068] According to the formula requirements, add 10 parts of active substances and 8 parts of Nongru 2201 respectively # , 4 servings of agricultural milk 600 # , 10 parts of methanol, and 68 parts of xylene are mixed evenly, and if necessary, they are heated and dissolved in a hot water bath to obtain emulsifiable concentrate.
[0069] Preparation of 25% wettable powder
[0070] According to the formula requirements, 25 parts of active substances, 2 parts of alkane amido taurate, 8 parts of methylnaphthalenesulfonic acid formaldehyde condensate, 6 parts of dibutylnaphthalenesulfonic acid formaldehyde condensate, 10 parts of white carbon black, 49 Parts of light calcium carbonate, etc. are fully mixed, and after being pulverized by an ultra-fine pulverizer, the processed product is obtained.
[0071] Preparation of 20% water emu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com