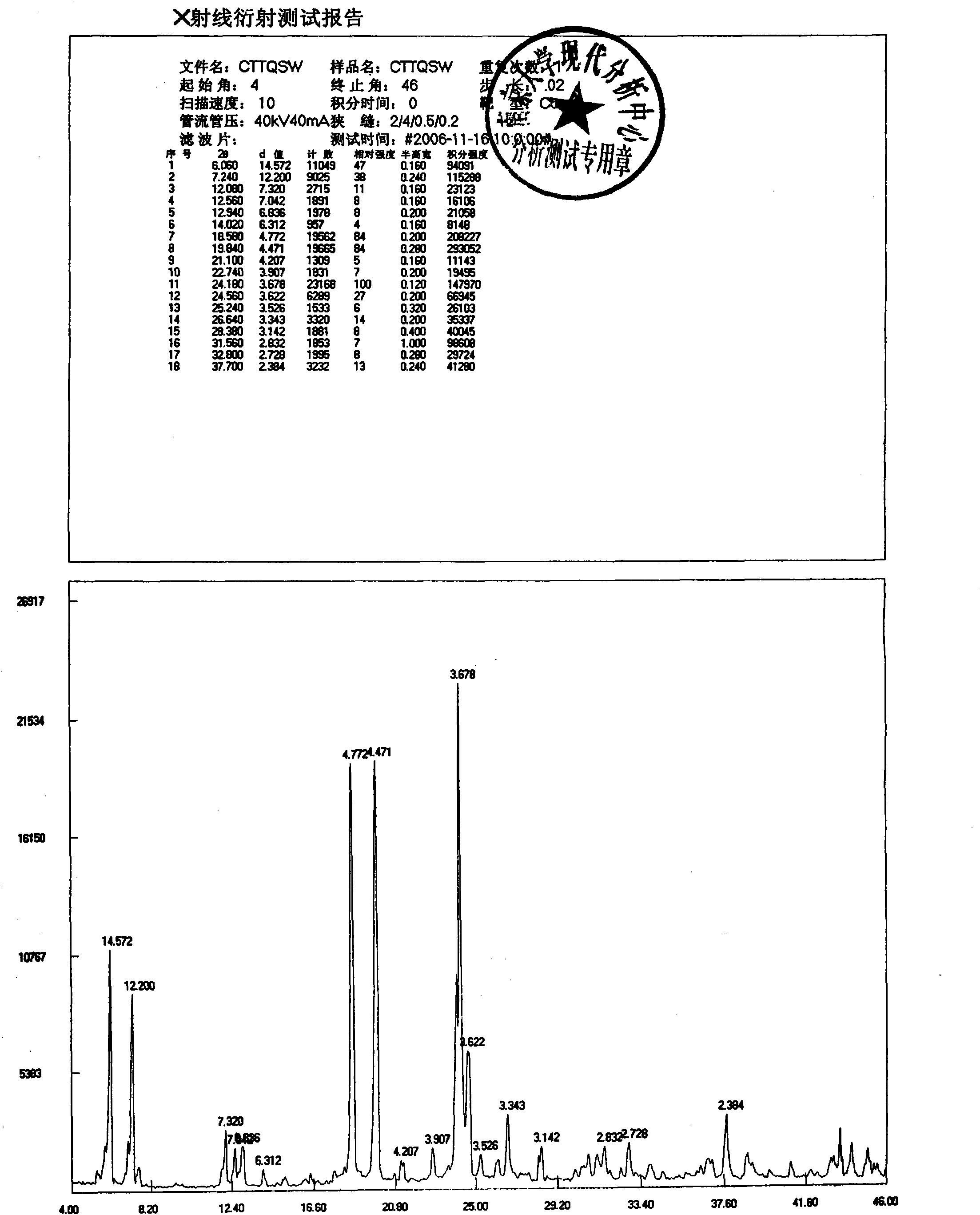
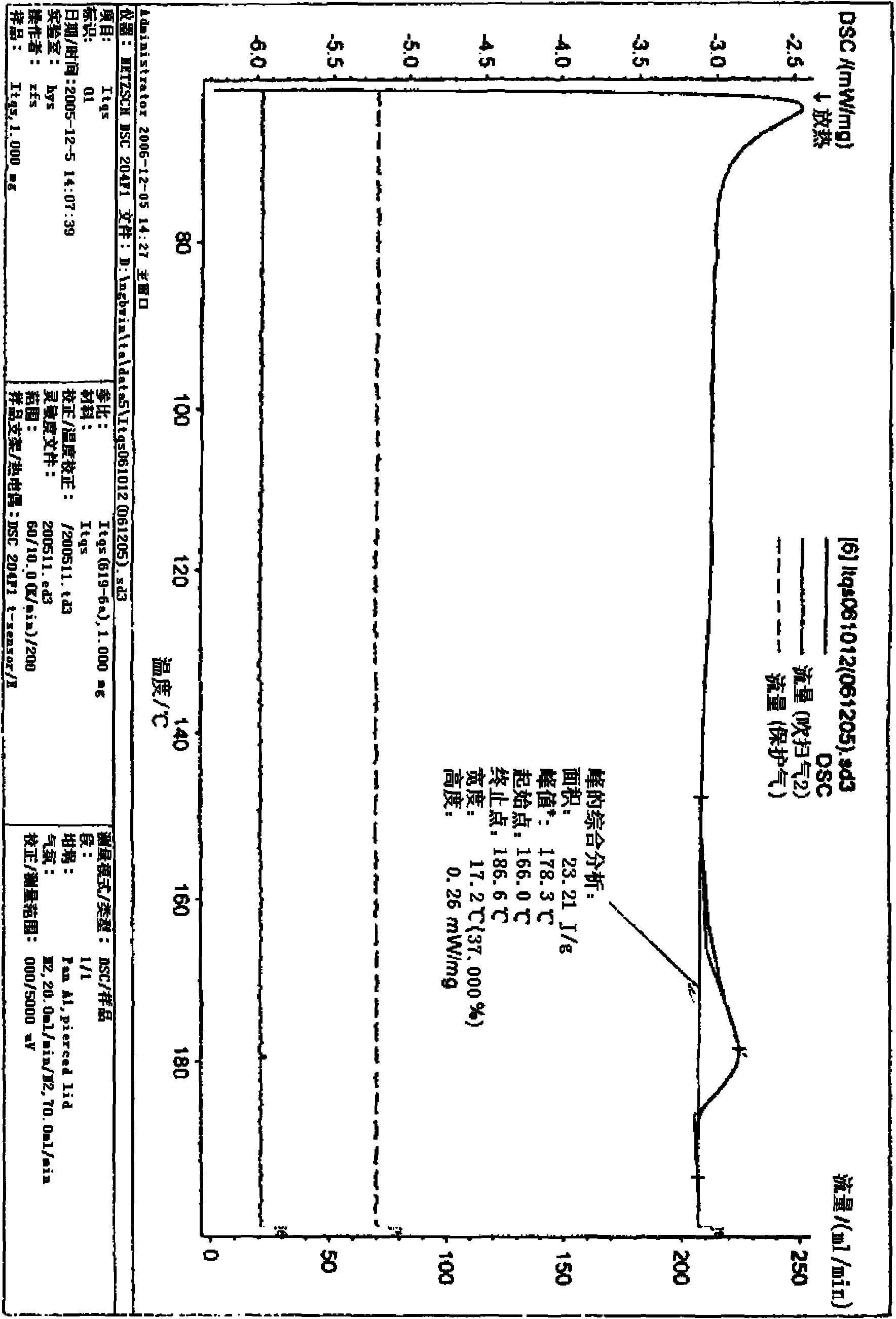
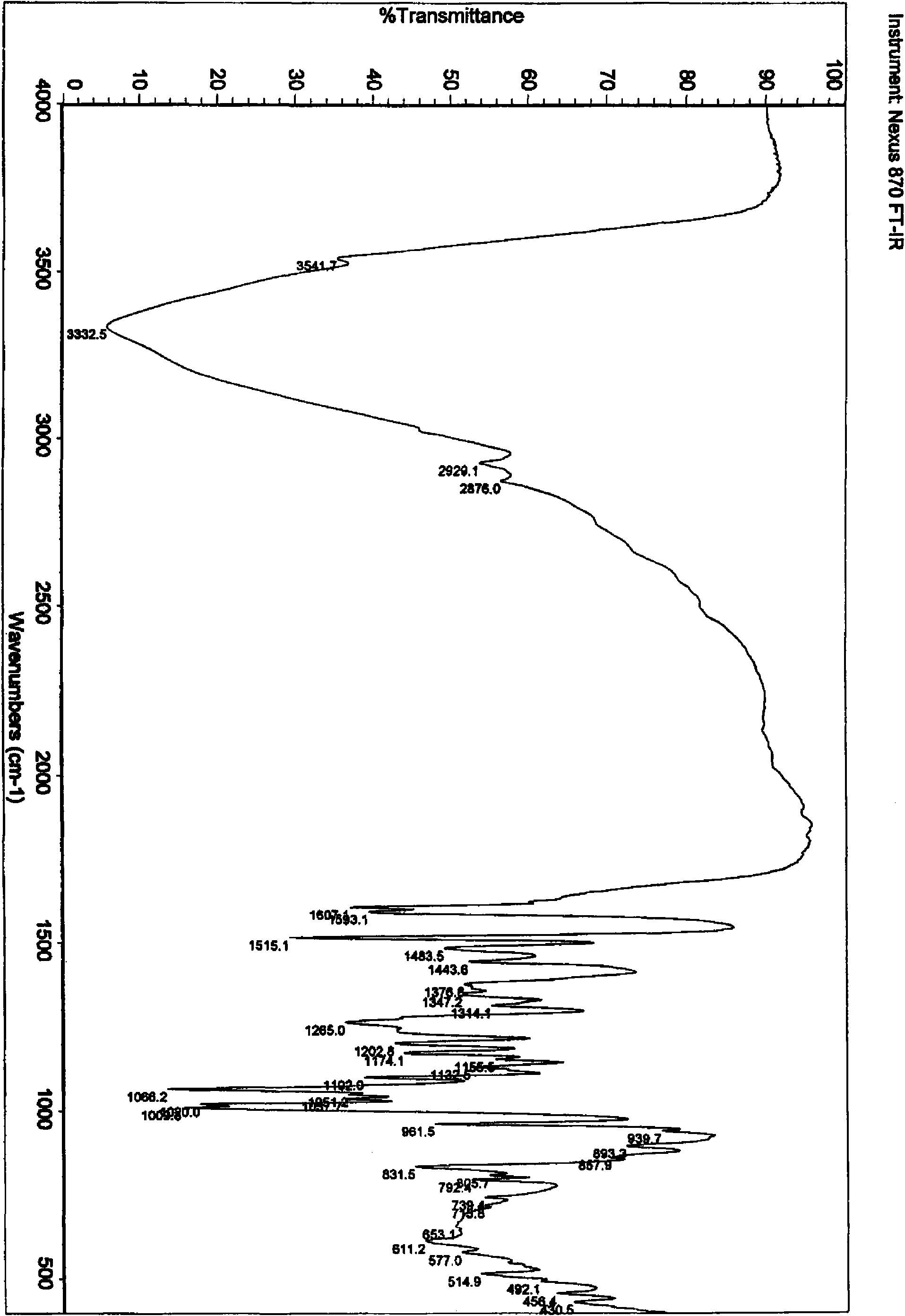
Stilbene glycoside derivative
A stilbene glycoside and ethanol technology, applied in the field of medicine, can solve the problems such as the purity of the stilbene glycoside far from meeting the quality requirements, the inability to meet the industrialized production, the cumbersome process and the like, and achieve the improvement of large dosage, less impurities and drug efficacy. exact effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Take 100kg of dried Polygonum multiflorum, cut into thin slices of 1-3mm, put it into a heat reflux extraction tank, add 10 times the amount of water and an appropriate amount of sodium sulfite, adjust the pH of the extract to 7.6, heat and reflux extraction 3 times, 1 hour each time, Combine the extracts, filter, and collect the filtrate. Pass the filtrate through a HPD-400 macroporous adsorption resin column, add 500L of water to elute, then add 80% ethanol to elute, collect the eluate containing stilbene glycosides, a total of 200L, concentrate under reduced pressure at 60°C to 10L, add ethyl acetate The ester was extracted 3 times, each time using 10 L, and the ethyl acetate solution was concentrated under reduced pressure at 60°C to dryness to obtain 2.5 kg of the crude stilbene glycoside extract.
[0050] Take the above crude extract, add 5.0L of ethanol, heat and stir to make it fully dissolved, filter, and pass the filtrate through a 200-300 mesh neutral alumina...
Embodiment 2
[0053] Take 100kg of dried Radix Polygoni Multiflori, crush it into a coarse powder, put it into a heat reflux extraction tank, add 8 times the amount of water and an appropriate amount of sodium bisulfite, adjust the pH of the extract to 7.5, heat and reflux extraction twice, each time for 2 hours, Combine the extracts, filter, and collect the filtrate. Pass the filtrate through HPD-100 macroporous adsorption resin column, add 600L of water to elute, add 70% ethanol to elute, collect the eluate containing stilbene glycosides, total 300L, concentrate under reduced pressure at 70°C to 15L, add n-butyl Alcohol extraction was carried out twice, with 15 L each time, concentrated under reduced pressure at 80°C, and concentrated n-butanol to dryness to obtain 2.3 kg of crude stilbene glycoside extract.
[0054] Take the above crude extract, add 8.0 L of ethyl acetate, heat and stir to make it fully dissolved, filter, pass the filtrate through a 200-300 mesh silica gel chromatography...
Embodiment 3
[0057] Take 120kg of dried Polygonum multiflorum, crush it into coarse powder, put it into a hot reflux extraction tank, add 7 times the amount of water and an appropriate amount of sodium carbonate, adjust the pH of the extract to 7.8, heat and reflux for 3 times, each time for 1.5 hours, and combine the extractions liquid, filtered, and the filtrate collected. Pass the filtrate through the AB-8 macroporous adsorption resin column, add 600L of water to elute, then add 90% ethanol to elute, collect the eluate containing stilbene glycosides, a total of 320L, concentrate under reduced pressure at 70°C to 20L, add ethyl acetate The ester was extracted 3 times with 20 L each time, concentrated under reduced pressure at 80°C, and the ethyl acetate was concentrated to dryness to obtain 2.92 kg of crude stilbene glycoside extract.
[0058] Take the above crude extract, add 12L of acetone, heat and stir to fully dissolve, filter, and pass the filtrate through a 200-300 mesh neutral al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com