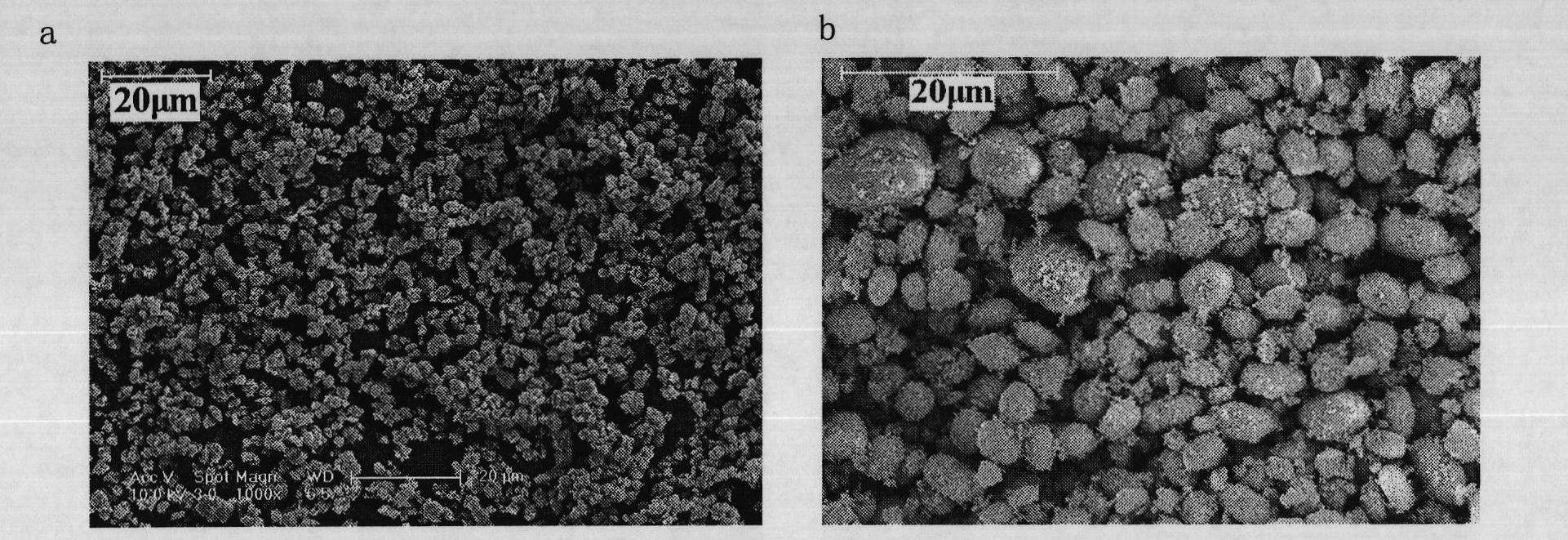
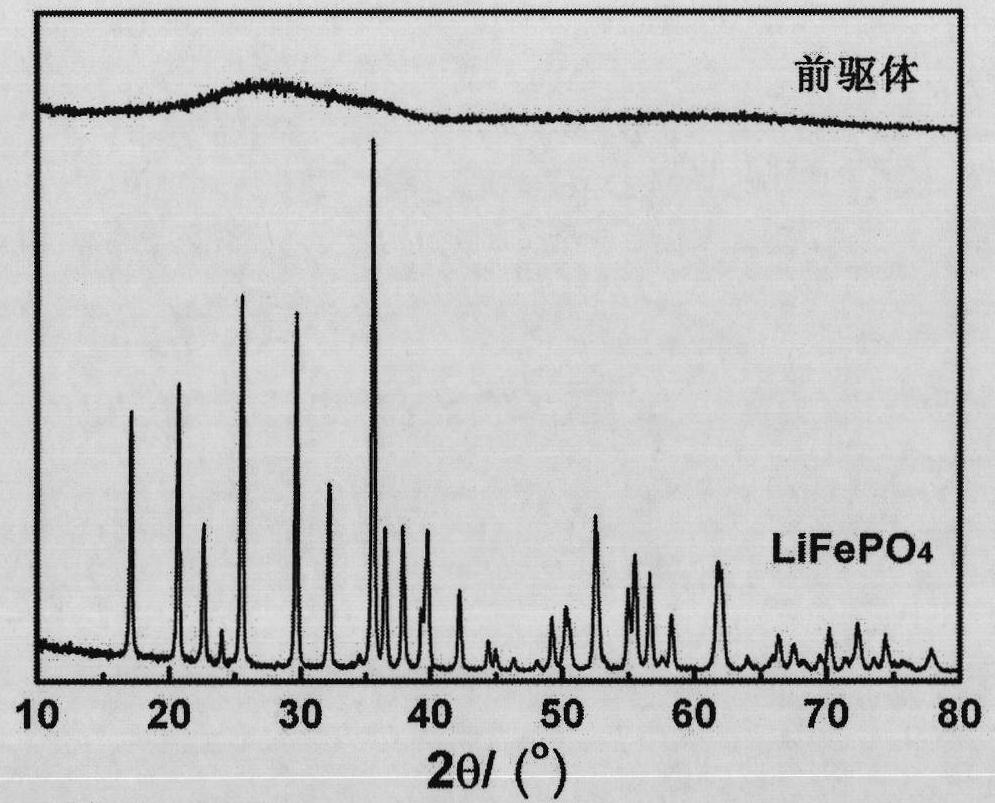
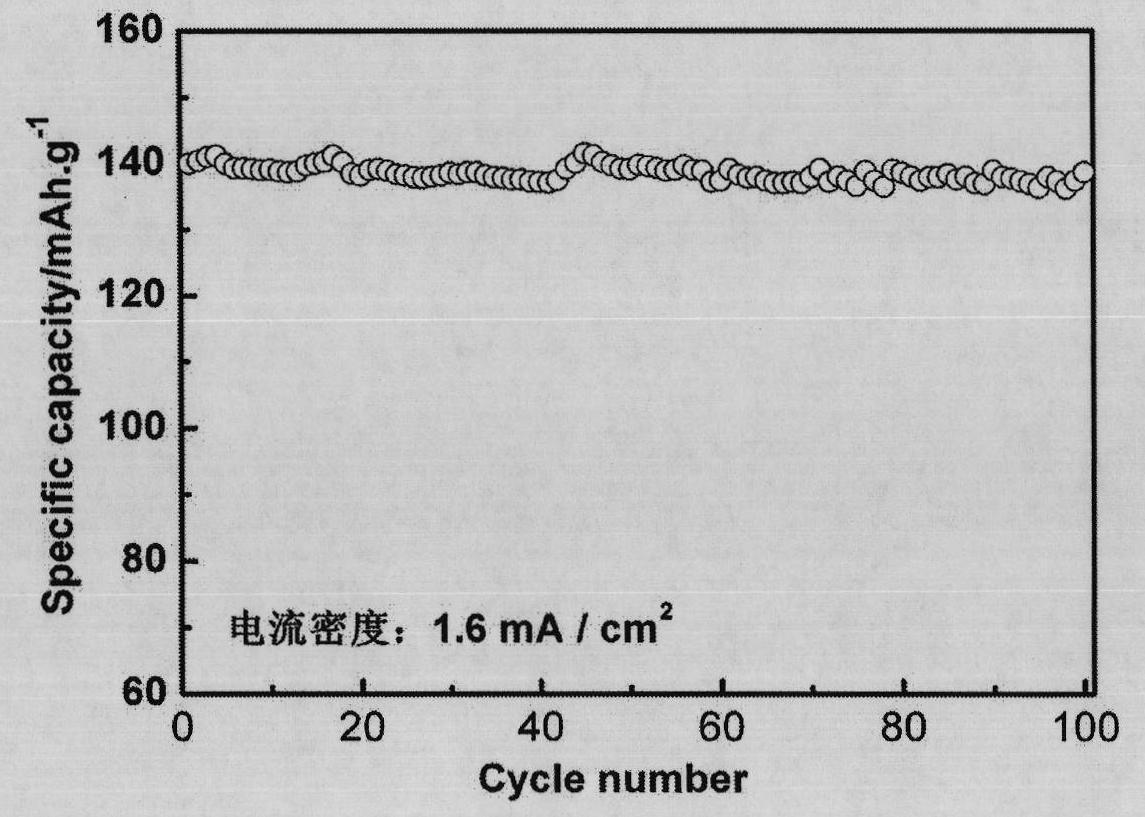
Lithium iron phosphate and ferrous phosphate, and preparation methods thereof
A technology of ferrous phosphate and lithium iron phosphate, which is applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc. Control the morphology, the crystal is easy to grow larger and other problems, to achieve the effect of suitable for large-scale production, low synthesis cost, and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1) prepare the mixed solution of ferrous sulfate and phosphoric acid, wherein the concentration of ferrous sulfate is 1.5mol / L, and the concentration of phosphoric acid is 1mol / L; Configuration concentration is the sodium hydroxide solution of 3mol / L; Under stirring condition, use The mixed solution of ferrous sulfate and phosphoric acid and sodium hydroxide solution are continuously input into the reactor with a volume of 1.5L by the metering pump respectively, and the flow rate of the mixed solution of ferrous sulfate and phosphoric acid is controlled at 6mL / min. The flow rate of 5L / min is fed into nitrogen; the flow rate of sodium hydroxide solution is adjusted so that the pH value of the reaction liquid in the reactor is 6.5±0.05; the temperature of the reaction liquid in the reactor is 50°C; the mixed material in the reactor is reacted The overflow port of the reactor is naturally overflowed and discharged; stop feeding after 20 hours of continuous feeding, and disc...
Embodiment 2
[0025] 1) prepare the mixed solution of ferrous chloride and potassium phosphate, wherein the concentration of ferrous chloride is 3mol / L, and the concentration of potassium phosphate is 2mol / L; Configuration concentration is the potassium hydroxide solution of 6mol / L; Next, the mixed solution of ferrous chloride and potassium phosphate, and potassium hydroxide solution were continuously input into a reactor with a volume of 1.5 L with a metering pump, and the flow rate of the mixed solution of ferrous chloride and potassium phosphate was controlled at 3 mL / min. , while feeding argon into the reactor at a flow rate of 20 L / min; adjusting the flow rate of the potassium hydroxide solution so that the pH value of the reaction solution in the reactor is 6.0 ± 0.05; the temperature of the reaction solution in the reactor is 30 ° C; The mixed material in the reactor is naturally overflowed and discharged through the overflow port of the reactor; stop feeding after 20 hours of continu...
Embodiment 3
[0029] 1) prepare the mixed solution of ferrous sulfate and sodium phosphate, wherein the concentration of ferrous sulfate is 0.15mol / L, the concentration of sodium phosphate is 0.1mol / L; Configuration concentration is the sodium hydroxide solution of 0.1mol / L; Stir Under the conditions, the mixed solution of ferrous sulfate and sodium phosphate, sodium hydroxide solution are continuously input into the reactor with a volume of 1.5L respectively with a metering pump, and the flow of the mixed solution of ferrous sulfate and sodium phosphate is controlled at 10mL / min. At the same time, feed nitrogen into the reactor at a flow rate of 0.1L / min; adjust the flow rate of the sodium hydroxide solution so that the pH value of the reaction solution in the reactor is 7.0 ± 0.05; the temperature of the reaction solution in the reactor is 80 ° C; The mixed material in the reactor is naturally overflowed and discharged through the overflow port of the reactor; stop feeding after 20 hours o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com