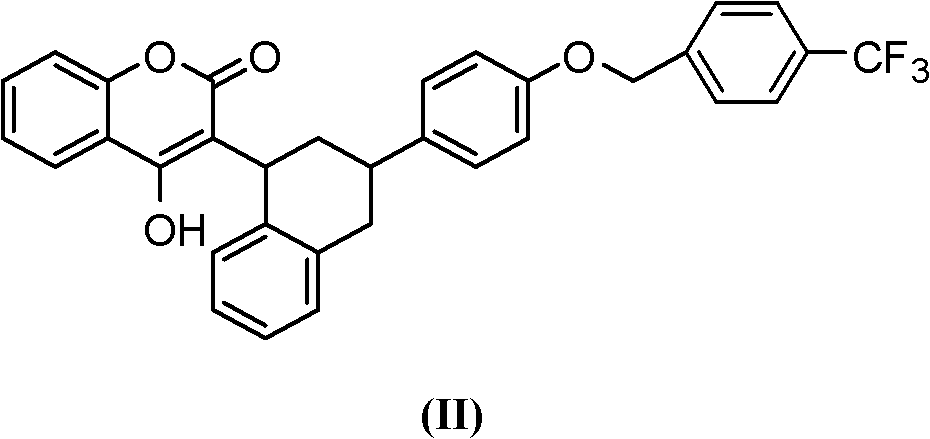
Method for synthesizing flocumafen intermediate
A synthesis method and an intermediate technology, which are applied in the field of synthesis of the anticoagulant rodenticide flumarinone intermediate, can solve the problems of increased cost, harsh reaction conditions, and large pollution, and achieve the avoidance of harsh requirements, simple and effective method, and The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
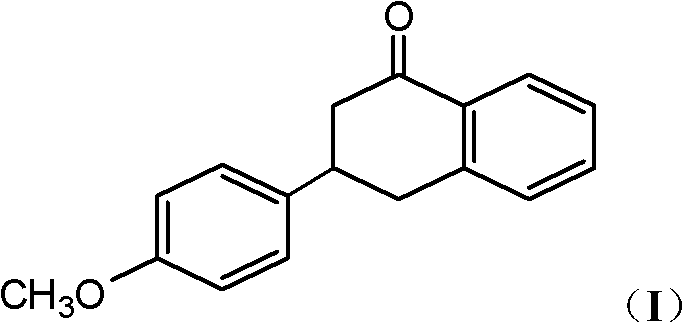
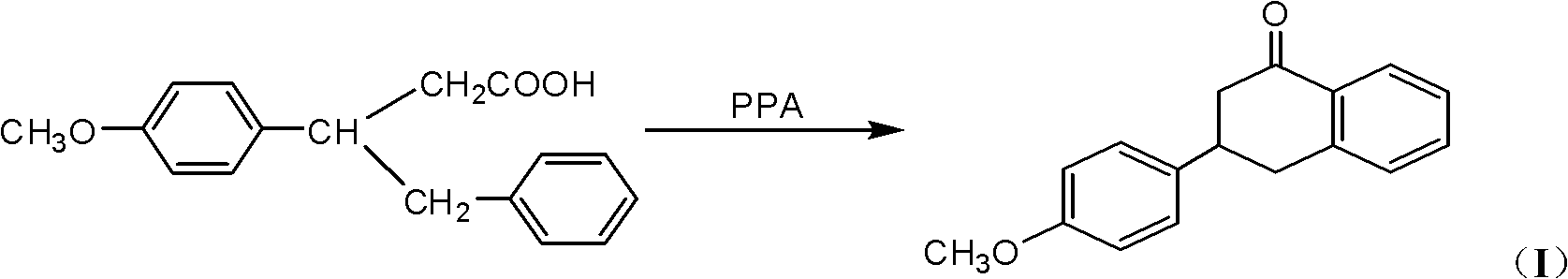
[0035] Take 10.8g of 3-benzyl-3-p-methoxyphenylpropionic acid, dissolve it in 250mL of toluene to obtain a mixed solution, add 15.8g of benzenesulfonic acid to the mixed solution, and reflux for 12h. After the reaction is completed, the organic solvent is evaporated to obtain Residue, after cooling the residue to room temperature, add 60 mL of ethyl acetate to dissolve, then wash with water and 10% sodium carbonate solution in sequence, separate the organic phase, evaporate the solvent in the organic phase, and finally recrystallize with methanol , 7.2 g of 3-p-methoxyphenyl-1,2,3,4-tetralin-1-one were obtained, with a yield of 71%. mp (melting point) 102-105°C.
[0036] 1 HNMR (500MHz, CDCl 3 )δ: 2.80~3.00(m, 2H), 3.18~3.51(m, 3H), 3.83(s, 3H), 6.93(d, J=8.5Hz, 2H), 7.24~7.55(m, 5H), 8.10 (d, J = 8.0 Hz, 1H).
Embodiment 2
[0038]Take 10.8g of 3-benzyl-3-p-methoxyphenylpropionic acid, dissolve it in 300mL of dichloroethane, add 4.6g of trifluoroacetic acid, and reflux for 20h. After the reaction is completed, the organic solvent is evaporated to obtain a residue. The mixture was cooled to room temperature, dissolved in 100 mL of ethyl acetate, and washed with water and 10% sodium carbonate solution in sequence. Separate the organic phase, evaporate the solvent in the organic phase, and recrystallize methanol to obtain 6.6 g of 3-p-methoxyphenyl-1,2,3,4-tetrahydronaphthalene-1-one with a yield of 65%. . mp 101-103°C.
[0039] Analysis data is with embodiment 1.
Embodiment 3
[0041] Take 10.8g of 3-benzyl-3-p-methoxyphenylpropionic acid, dissolve it in 250mL of xylene, add 19.6g of trichloroacetic acid, and reflux for 5h. After the reaction is completed, the organic solvent is evaporated to obtain a residue, and the residue is cooled After reaching room temperature, 100 mL of ethyl acetate was added to dissolve, followed by washing with water and 10% sodium carbonate solution. Separate the organic phase, evaporate the solvent in the organic phase, and recrystallize methanol to obtain 7.0 g of 3-p-methoxyphenyl-1,2,3,4-tetrahydronaphthalene-1-one with a yield of 69%. . mp 101-103°C.
[0042] Analysis data is with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com