Bifunctional glutathione synthetase and method for producing glutathione by using same
A technology of glutathione and synthetic enzymes, applied in the direction of microorganism-based methods, biochemical equipment and methods, enzymes, etc., to achieve the effect of improving production levels and improving production levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 utilizes actinobacillus succinic acid to synthesize glutathione
[0042] 1. Culture of Actinobacillus succinogenes
[0043] Put Actinobacillus succinogenes 130Z (CICC 11014) from the glycerol tube stored at -20°C into the fermentation medium at an inoculum size of 1%. Na 2 HPO 4 12H 2 O 0.5g, NaH 2 PO 4 2H 2 O 0.5g, pH 6.5, 37°C, 220rpm, shaking culture for 24 hours.
[0044] 2. Cell pretreatment
[0045] The cultivated Actinobacillus succinici culture solution was centrifuged at 8000rpm for 5min to collect the cells, washed and centrifuged three times with 0.05mol / L, pH7.0 phosphate buffer under the same conditions. Cells were frozen at -20°C for 2 hours and permeabilized.
[0046] 3. Glutathione synthesis reaction
[0047] Weigh 2g of permeabilized wet bacteria and add to 20mL reaction solution, the reaction solution is 0.2mol / L (pH7.0) potassium phosphate buffer solution, containing 40mmol / L L-glutamic acid, L-cysteine 20mmol / L, glycine 40mmol...
Embodiment 2
[0048] Embodiment 2 utilizes bacillus cereus to synthesize glutathione
[0049] Put Bacillus cereus (CGMCC 1.932) from a glycerol tube stored at -20°C into the fermentation medium at an inoculum size of 1%. 220rpm, shaking culture for 12 hours, permeabilization treatment and glutathione synthesis reaction were carried out on Bacillus cereus according to the method described in Example 1, after 8 hours of reaction, glutathione could accumulate 163mg / L.
Embodiment 3
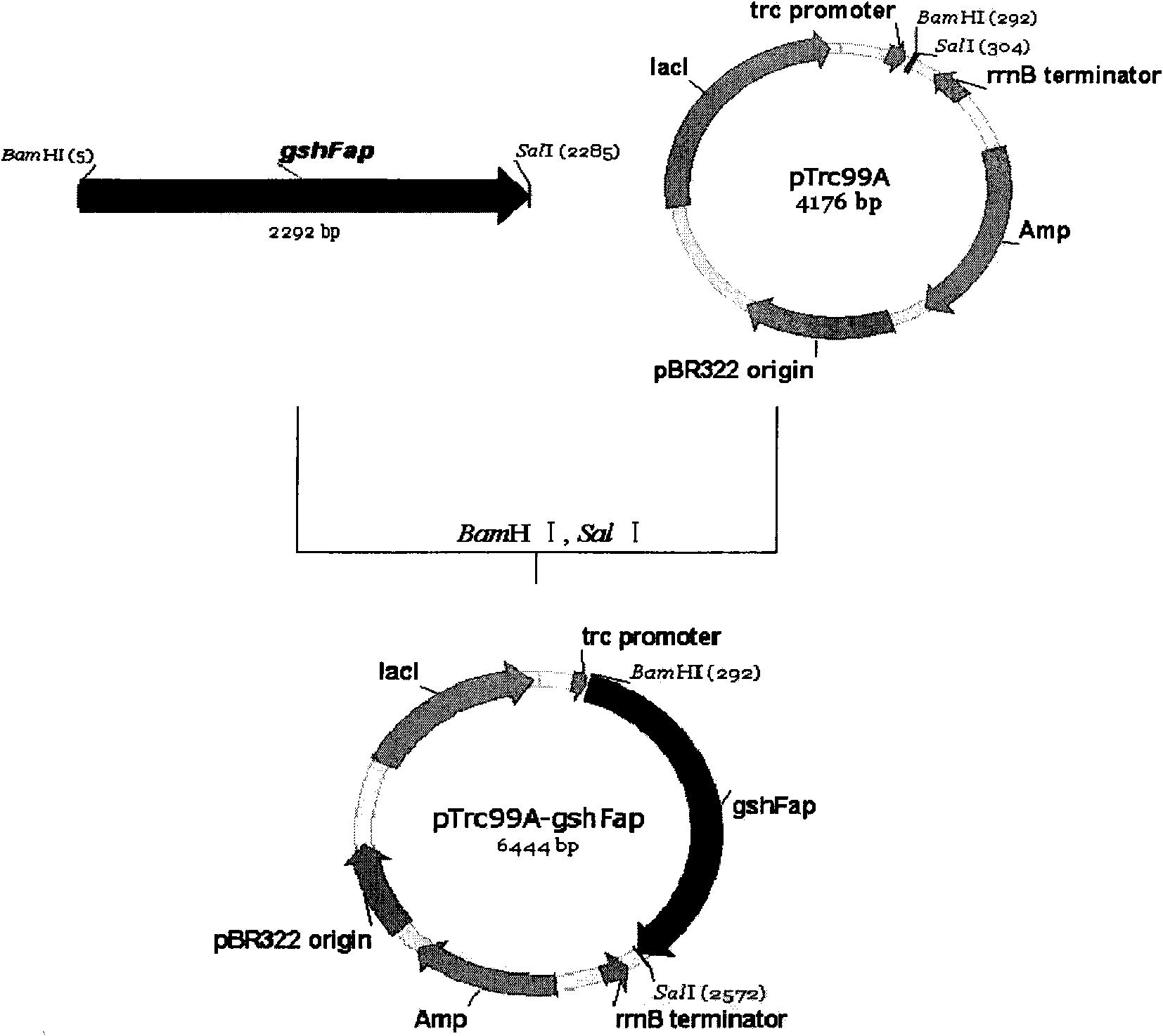
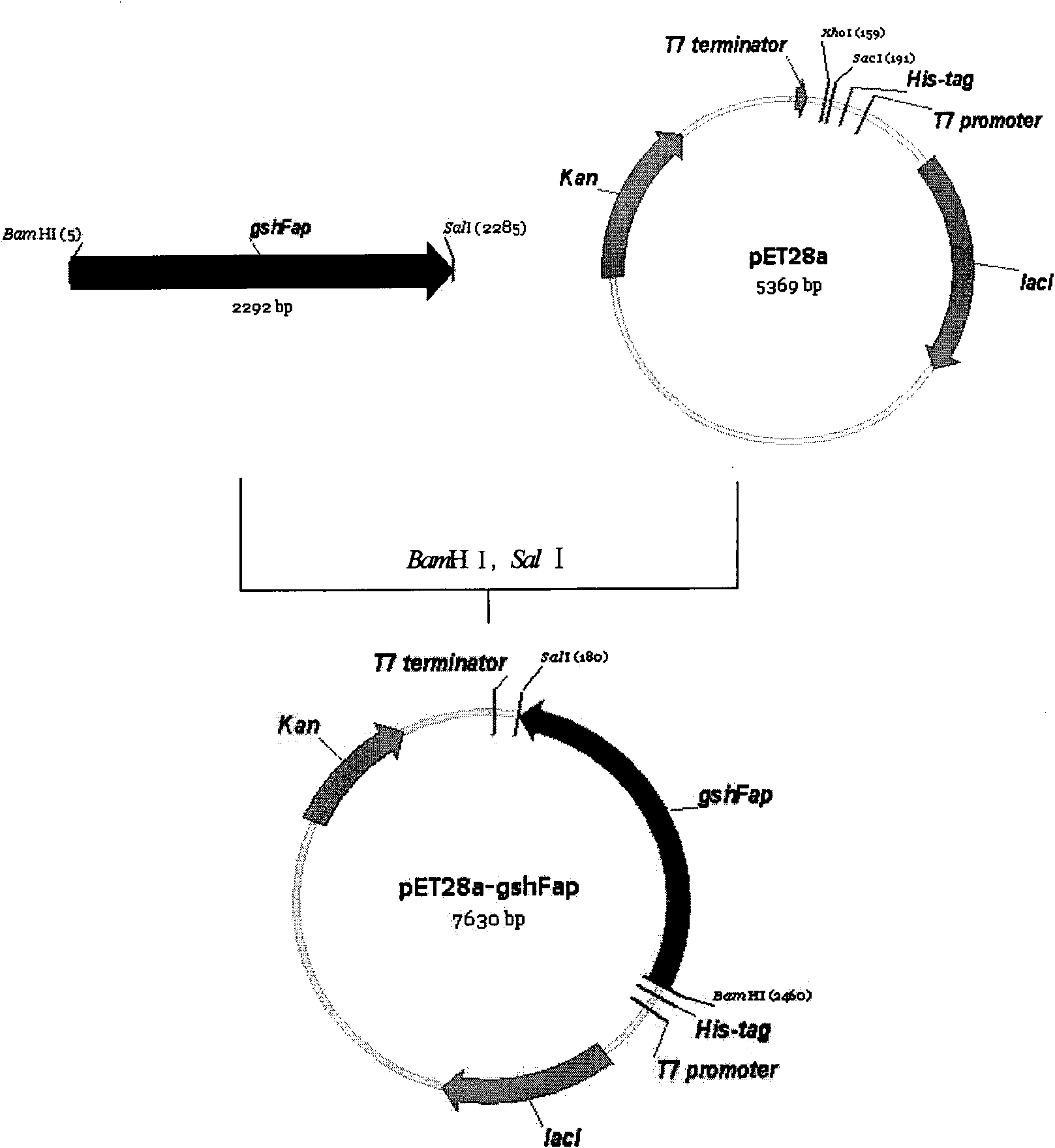
[0050] Example 3 Construction of Recombinant Escherichia coli JM109 (pTrc99a-gshFap)
[0051] 1. Extraction of Actinobacillus pleuropneumonia genomic DNA
[0052] Put Actinobacillus pleuropneumonia (CVCC 259) from the glycerol tube stored at -20°C into the fermentation medium at an inoculum size of 1%. The specific components are (1L contains): peptone 10.0g, yeast extract 5g, NaCl 10g, NAD 0.2 g. pH 7.4, 37°C, static culture for 24 hours, centrifuge at 13400×g, 4°C for 2 minutes, and collect the bacteria. The whole genome DNA of Actinobacillus pleuropneumonia was extracted by BBI Whole Genome DNA Extraction Kit.
[0053] 2. Cloning of the gshF gene of Actinobacillus pleuropneumoniae
[0054] The upstream primer is: 5'-GCGC GGATCC ATGAAATTACAACAAC-3', the underlined part is the restriction site of BamH I.
[0055] The downstream primer is 5'-GAT GTC GAC TTAAGGCAGTTCTGGGAA-3', the underlined part is the Sal I restriction site.
[0056] Using the whole genome of Actinoba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com