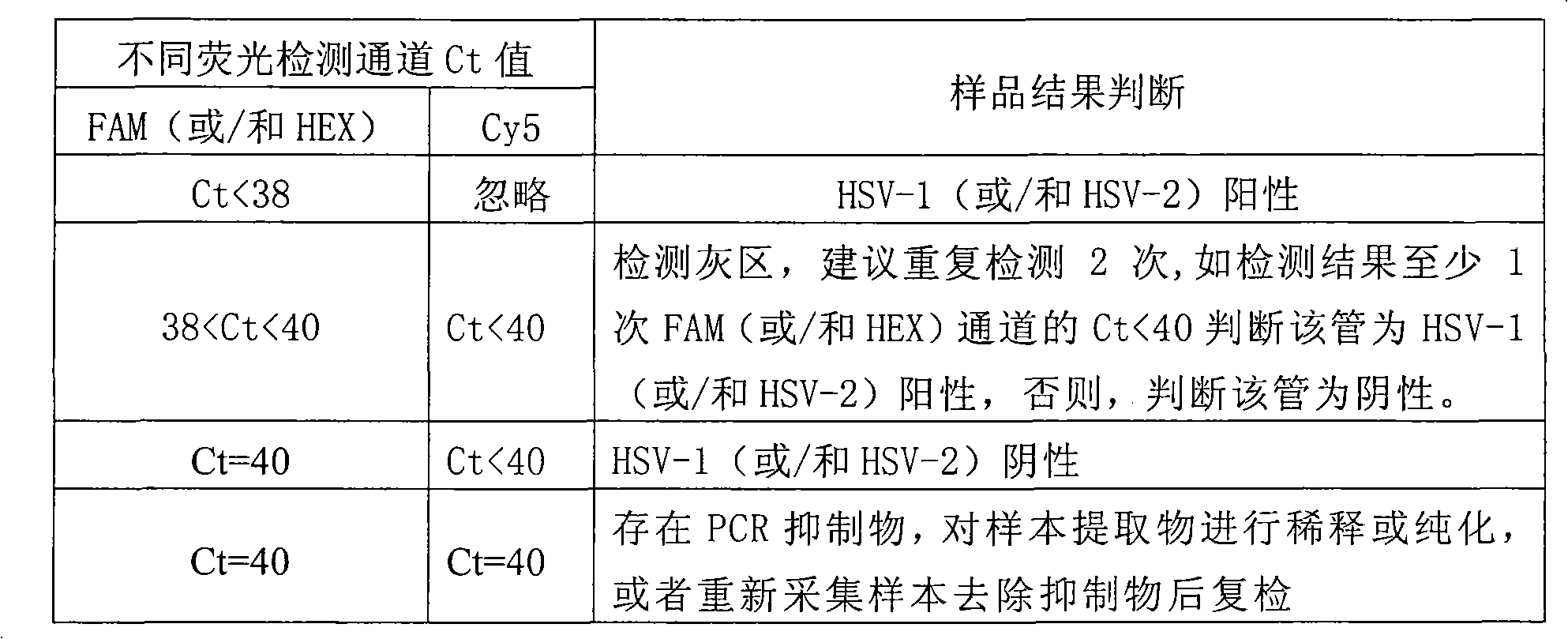
Method and kit for detecting genotypes of herpes simplex virus
A herpes simplex virus and genotyping technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, material stimulation analysis, etc., can solve problems such as indistinct melting curve characteristics, low market share, and inability to judge results , to achieve stable and reliable results, ensure specificity of detection, easy and fast operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: the preparation of herpes simplex virus genotyping detection kit (PCR-fluorescent probe method)
[0050] (1) Primer probe synthesis
[0051] Primer probes were designed according to the sequence of SEQ ID No.1 in the content of the invention, and a commercial sequence synthesis company (Shanghai Sangon Bioengineering Technology Service Co., Ltd.) was entrusted to synthesize the following sequences:
[0052] The upstream primer is 5'-TCCATgCgCATCATCTACg-3', as shown in SEQ ID No.2.
[0053] The downstream primer is 5'-TgTggCTCgCCATCTTgT-3', as shown in SEQ ID No.3.
[0054] The HSV-1 fluorescent probe is 5'-ACTCCATATTTgTgCTgTgCCgC-3' (5' end labeled FAM, 3' end labeled BHQ-1), as shown in SEQ ID No.4.
[0055] The HSV-2 fluorescent probe is 5'-TggCCACCAgCgCTTCgC-3' (HEX is marked at the 5' end, and BHQ-1 is marked at the 3' end), as shown in SEQ ID No.5.
[0056] The internal reference probe is 5'-TTCAgCgAgCgTgggACATCAgg-3' (5' labeled Cy5, 3' labeled BH...
Embodiment 2
[0077] Example 2: Application of Herpes Simplex Virus Genotyping Detection Kit (PCR-Fluorescent Probe Method) in Skin Oral Herpes HSV Detection
[0078] (1) Sample processing
[0079] Use a cotton swab to pick up herpes exudate from the patient's skin, eyes or oral mucosa, then put the cotton swab into 1ml of normal saline, shake it well, squeeze the cotton swab dry, and centrifuge the rinse solution at 13,000rpm for 6 minutes. Carefully aspirate the supernatant with a pipette (5 μl of residual liquid can be reserved to prevent aspiration of the precipitate). Add 50 μl of nucleic acid extraction solution to the pellet, vortex to mix, keep at 100°C for 10 minutes, centrifuge at 13,000 rpm for 6 minutes, and take the supernatant for fluorescent PCR amplification detection. The negative and positive controls of the kit are processed in the same way as the sample solution.
[0080] (2) Amplification detection
[0081] According to the method in the summary of the invention, eac...
Embodiment 3
[0083] Embodiment 3: the application of herpes simplex virus genotyping detection kit (PCR-fluorescent probe method) in the detection of genital herpes HSV
[0084] (1) Sample processing
[0085] Use a cotton swab to collect the secretions from the urethral lesion of the patient, then put the cotton swab into 1ml of normal saline, shake it well, squeeze the cotton swab dry, take the rinse solution and centrifuge it at 13,000rpm for 6 minutes, and use a pipette Carefully aspirate the supernatant (to prevent aspiration of the precipitate, 5 μl of residual liquid can be reserved). Add 50 μl of nucleic acid extraction solution to the pellet, vortex to mix, keep at 100°C for 10 minutes, centrifuge at 13,000 rpm for 6 minutes, and take the supernatant for fluorescent PCR amplification detection. The negative and positive controls of the kit are processed in the same way as the sample solution.
[0086] (2) Amplification detection
[0087] According to the method in the summary of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com