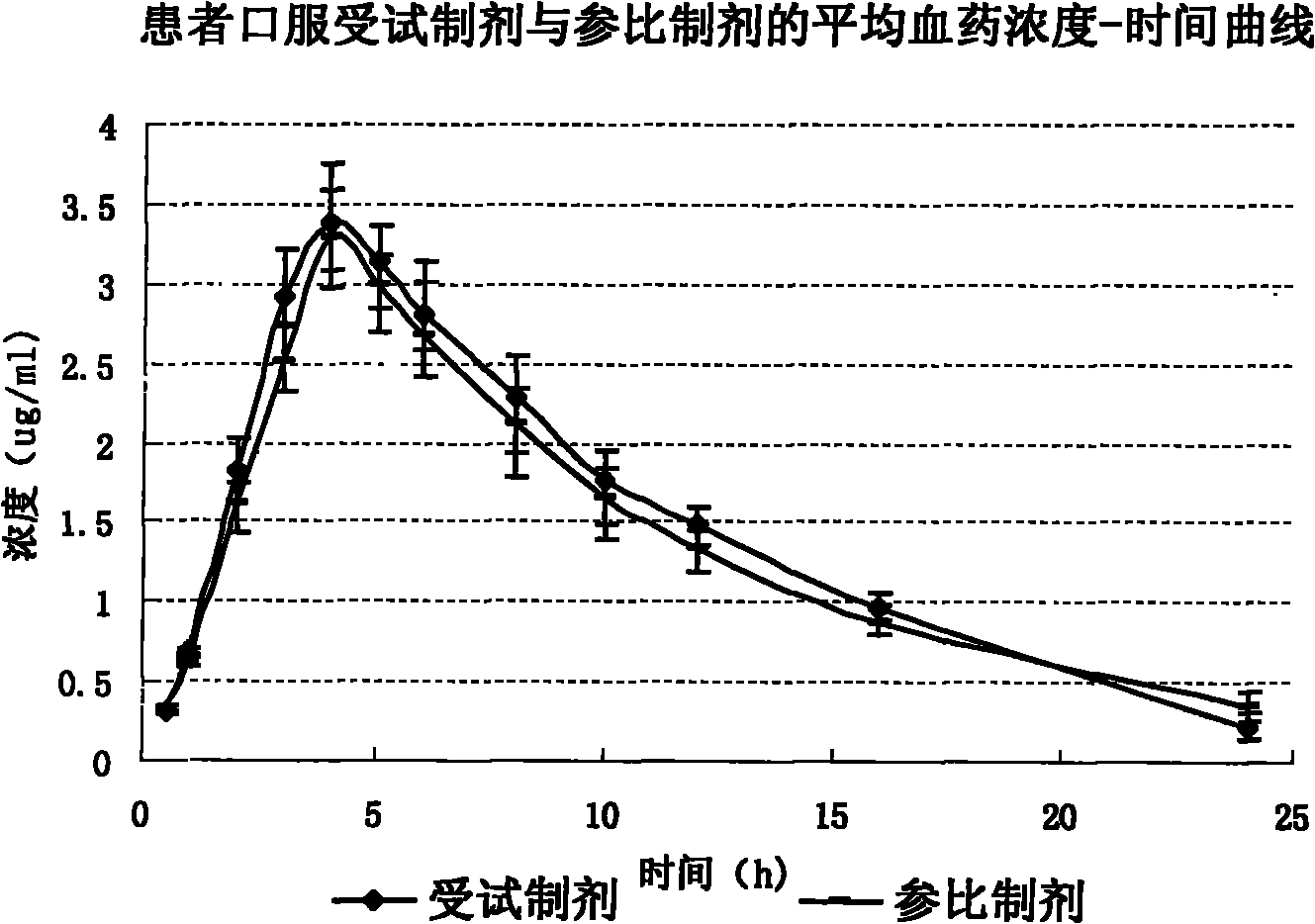
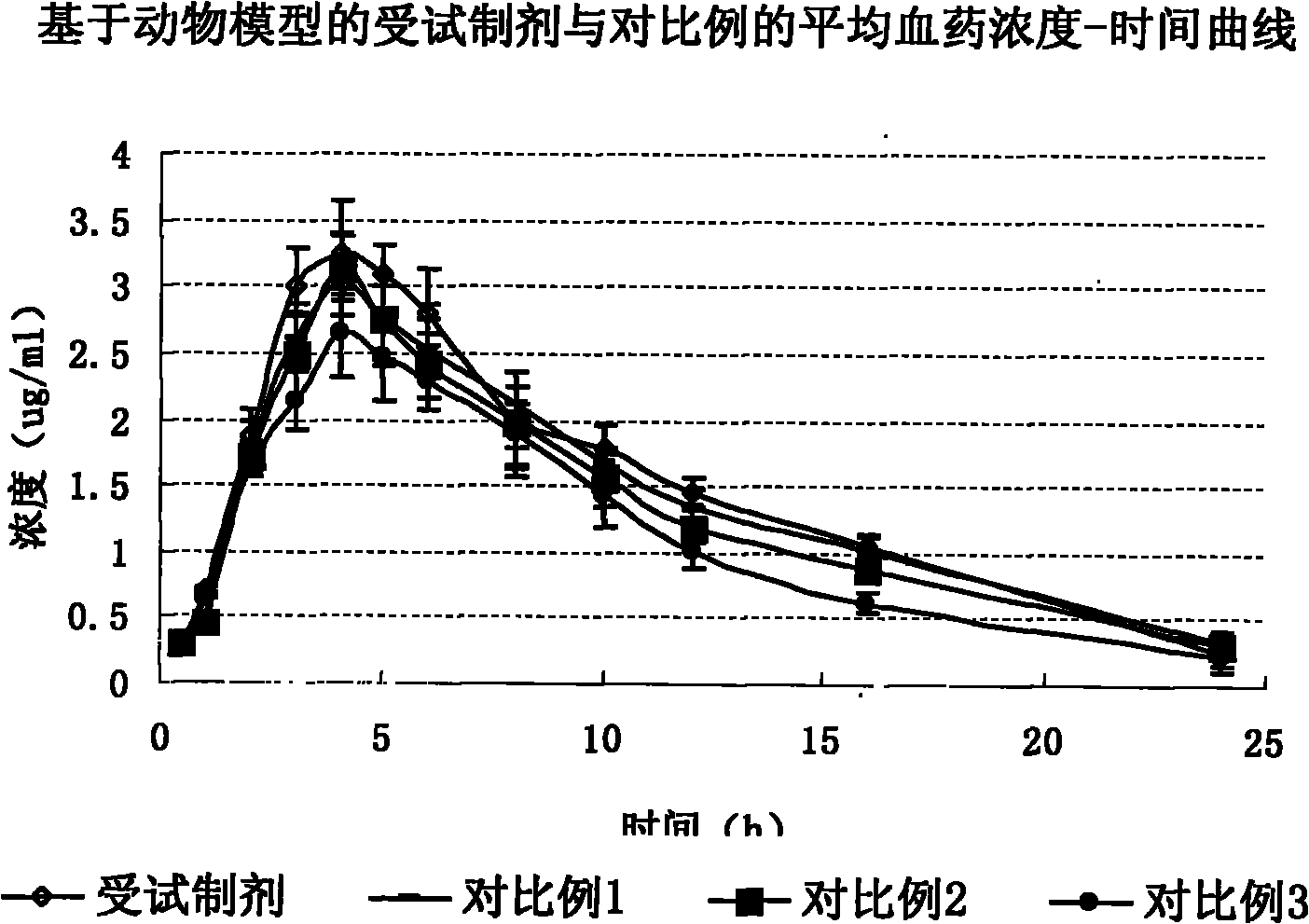
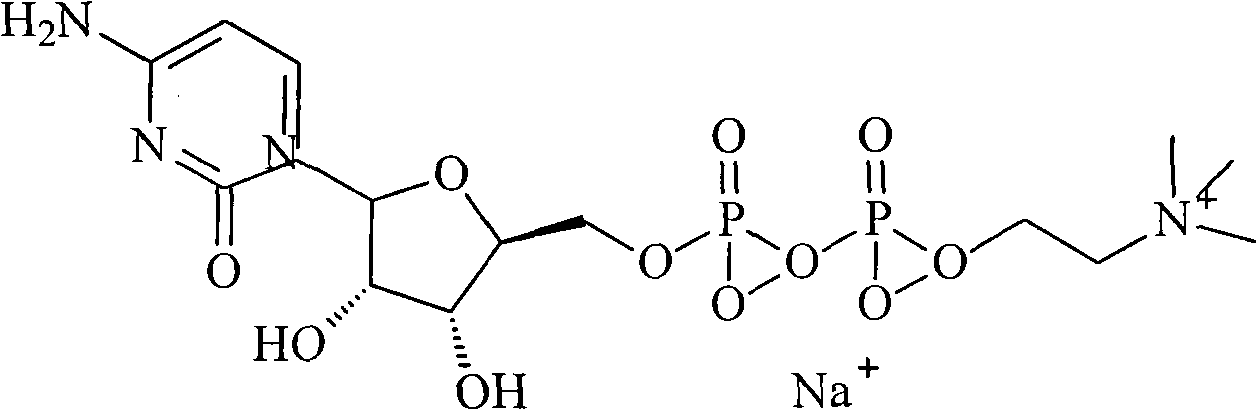
Citicoline sodium liposome solid preparation
A technology of citicoline sodium and liposome preparation, which is applied in the field of citicoline sodium liposome solid preparation and its preparation method, and achieves the effects of simple preparation process, improved product quality and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation of embodiment 1 citicoline sodium liposome
[0051] prescription:
[0052] Citicoline Sodium 100g
[0054] Dioleoylphosphatidylglycerol 300g
[0055] Sodium deoxycholate 40g
[0056] Octadecylamine 20g
[0057] Preparation Process:
[0058] (1) 100g egg yolk lecithin and 300g dioleoylphosphatidylglycerol and 40g sodium deoxycholate and 20g octadecylamine are dissolved in a mixed solvent of acetone and dichloromethane with a volume ratio of 1:1 in 2000ml, and placed in The organic solvent was removed under reduced pressure on a rotary thin film evaporator to obtain a phospholipid film;
[0059] (2) Add 1000 ml of acetic acid-sodium acetate buffer solution having a pH value of 5.3 dissolved with 100 g of citicoline sodium to fully hydrate the phospholipid film, mix the solution evenly, and heat it at 70° C. for 60 minutes of ultrasonic treatment;
[0060] (3) Spray-dry the solution obtained in the above step (2)...
Embodiment 2
[0082] The preparation of embodiment 2 citicoline sodium liposomes
[0083] prescription:
[0084] Citicoline Sodium 100g
[0086] Dioleoylphosphatidylglycerol 750g
[0087] Sodium deoxycholate 200g
[0088] Octadecylamine 100g
[0089] Preparation Process:
[0090] (1) 250g egg yolk lecithin and 750g dioleoylphosphatidylglycerol and 200g sodium deoxycholate and 100g octadecylamine are dissolved in a mixed solvent of acetone and dichloromethane with a volume ratio of 1:1 in 4000ml, placed in The organic solvent was removed under reduced pressure on a rotary thin film evaporator to obtain a phospholipid film;
[0091] (2) Add 2000 ml of acetic acid-sodium acetate buffer solution with 100 g of citicoline sodium at a pH value of 5.3 to fully hydrate the phospholipid film, mix the solution evenly, and conduct ultrasonic treatment for 60 minutes at a temperature of 50° C.;
[0092] (3) Spray-dry the solution obtained in the above step (2...
Embodiment 3
[0100] The preparation of embodiment 3 citicoline sodium liposome granules
[0101] Prescription (1000 bags)
[0102] Citicoline Sodium Liposome (calculated as Citicoline Sodium) 100g
[0103] Sucrose 650g
[0104] Mannitol 500g
[0105] Orange flavor 15g
[0106] Povidone K30 10g
[0107] Preparation Process:
[0108] (1) Pulverize the liposome containing 100g citicoline sodium, cross 80 mesh sieves, and set aside;
[0109] (2) Pulverize the sucrose, pass through an 80-mesh sieve, mix, and set aside;
[0110] (3) Mix 650g sucrose, 500g mannitol and 100g citicoline sodium liposome evenly, add 50% ethanol solution 200ml of 5% povidone K30 containing 15g orange essence to make soft material, cross 20 mesh sieves and granulate , dried at 60°C, granulated through a 18-mesh sieve;
[0111] (4) Subpackage the dried granules to obtain citicoline sodium liposome granules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com