Fluorinated triphenylamine derivative biological blue light materials and preparation method thereof
A technology of blue light materials and triphenylamine, which is applied in the direction of luminescent materials, preparation of amino compounds, organic compounds, etc., can solve the problems of restricting the development of full-color devices, narrow selection of transmission layer materials, and short life of green light materials. The preparation method is simple, the operation is convenient, and the price is reasonable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
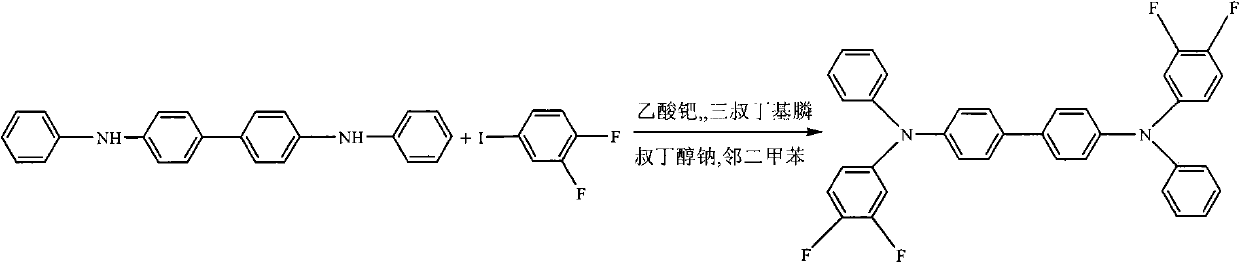
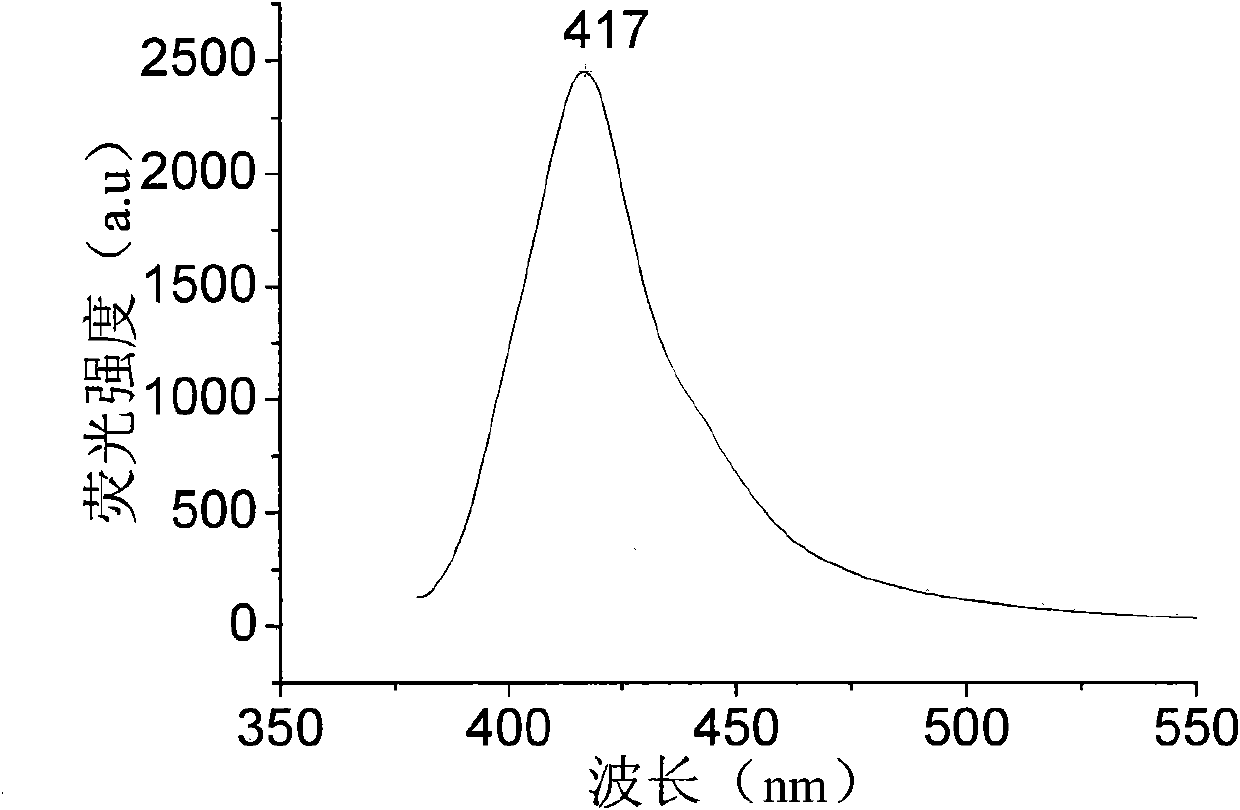
[0030] Synthetic routes such as figure 2 shown. In a 150mL flask, 2.016g (6mmol) N, N'-diphenyl-[1,1'-biphenyl]-4,4'-diamine, 5.9mg (0.026mmol) palladium acetate, 1.382g (14.4mmol) sodium tert-butoxide, then add 60mL o-xylene, 1.7mL (14.4mmol) 3,4-difluoro-1-iodobenzene, 21mg (0.104mmol) tri-tert-butylphosphine, under nitrogen protection, 135 The reaction was carried out at constant temperature at ℃ for 12 hours. After the reaction is completed and cooled, add 60mL of distilled water, stir for 30 minutes, filter off the insoluble matter, then use a separating funnel to separate the water layer from the organic layer, and the organic layer is filtered through anhydrous MgSO 4 After drying, the solvent was evaporated to obtain a crude product. The crude product was purified by column chromatography and recrystallized from ethanol to obtain N,N'-diphenyl-N,N'-bis(3,4-difluorophenyl)-[1,1'-biphenyl]- 4,4'-Diamine, 73% yield. Solid photoluminescence (thin film, excitation wav...
Embodiment 2
[0035] Synthetic routes such as Figure 4 shown. Others are the same as embodiment 1, and the polyfluorobenzene derivative used is 2,4-difluoro-1-iodobenzene, and the catalyst used is three (dibenzylidene acetone) dipalladium (Pd 2 (dba) 3 ) and 1,1'-bis(diphenylphosphino)ferrocene (dppf), a fluorine-substituted triphenylamine derivative N,N'-diphenyl-N,N'-bis(2, 4-difluorophenyl)-[1,1'-biphenyl]-,4'-diamine, others are similar to Example 1. The yield was 61%.
Embodiment 3
[0037] Synthetic routes such as Figure 5 shown. Others are the same as embodiment 1, and the polyfluorobenzene derivative used is 2,3-difluoro-1-iodobenzene, and the catalyst used is three (dibenzylidene acetone) dipalladium (Pd 2 (dba) 3 ) and tri-tert-butylphosphine, the inert gas used is argon, and a fluorine-substituted triphenylamine derivative N, N'-diphenyl-N, N'-bis(2,3-difluorophenyl )-[1,1'-biphenyl]-4,4'-diamine, others are similar to Example 1. The yield was 68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com