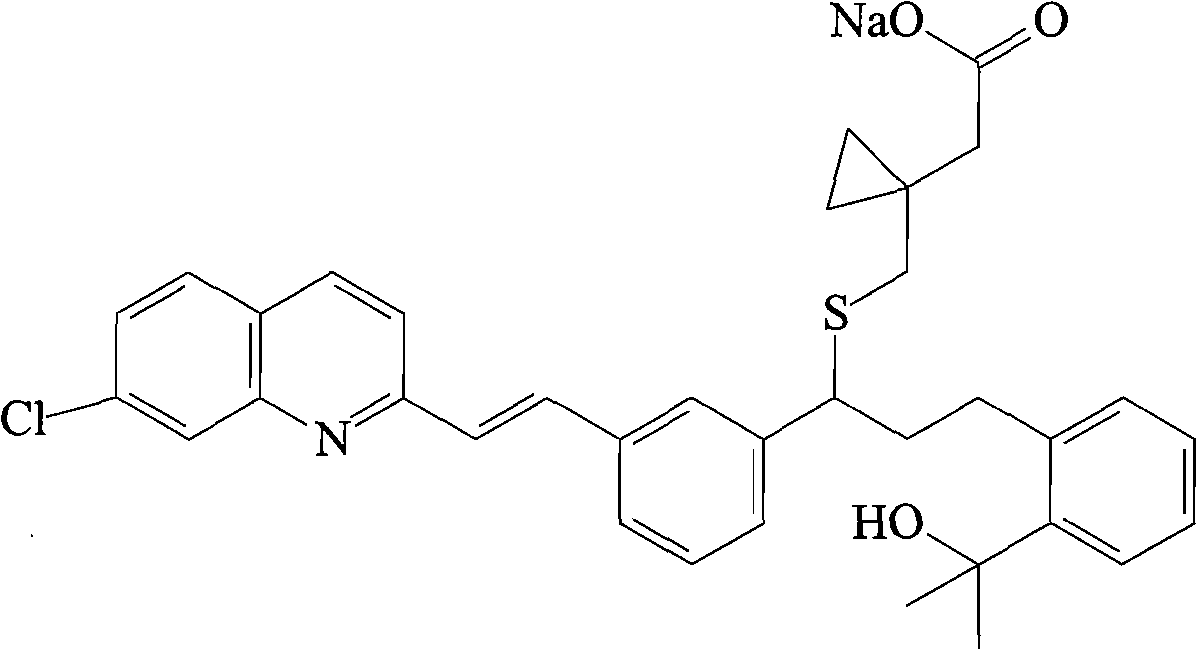
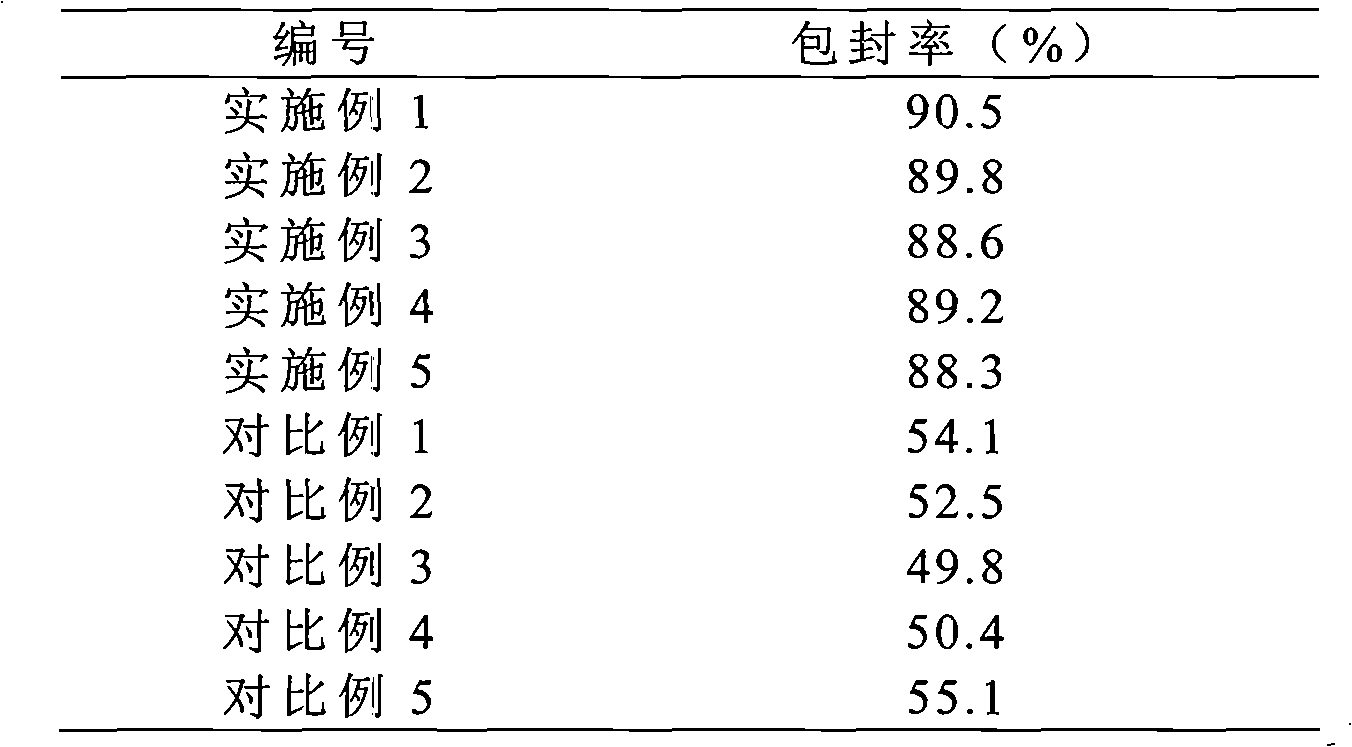
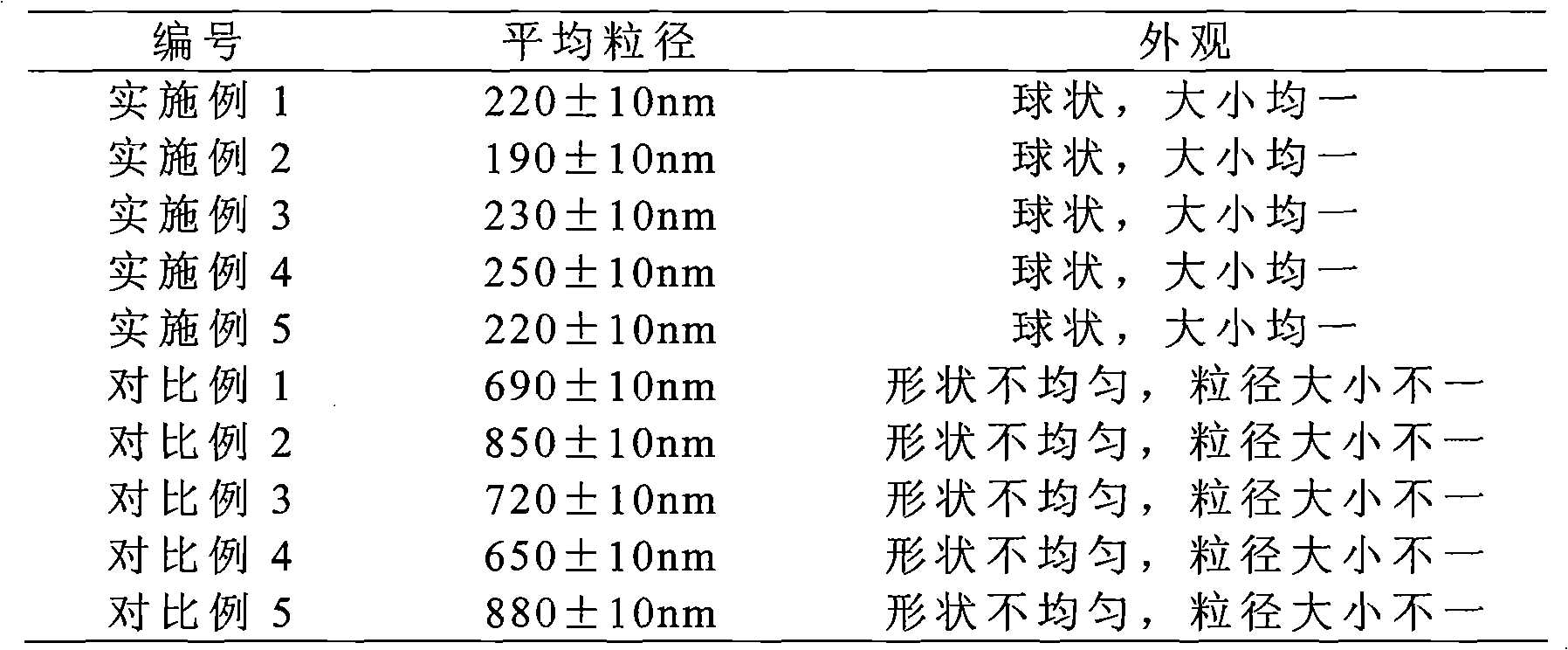
Montelukast sodium liposome solid preparation
A technology of montelukast sodium lipid and montelukast sodium, which is applied in the directions of liposome delivery, medical preparations of inactive ingredients, and drug combinations, etc., can solve the problem of poor liposome stability and encapsulation efficiency , high encapsulation rate and high bioavailability, to achieve the effect of improving storage, high encapsulation rate and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0072] On the other hand, the present invention provides the preparation method of above-mentioned montelukast sodium liposome, the method comprises the following steps:
[0073] (1) Dissolving hydrogenated soybean lecithin, cholesterol, Tween 80 and soybean sterol in isopropanol to obtain a phospholipid solution;
[0074] (2) Montelukast sodium and mannitol are dissolved in water, and the above-mentioned phospholipid solution is slowly dropped into it, while stirring evenly with a magnetic stirrer to obtain a liposome suspension containing isopropanol;
[0075] (3) Place the above-mentioned suspension in an ultrasonic container and sonicate to a translucent colloidal solution;
[0076] (4) Filter the above-mentioned translucent colloid with a microporous membrane, place the filtrate at -40~-45°C for 4-6 hours, and then slowly raise the temperature to -5°C at a rate of 2°C / hour, and keep it warm for 2 hour, and then heated up to 30° C. at a rate of 5° C. / hour, and dried for 4...
Embodiment 1
[0104] The preparation of embodiment 1 montelukast sodium liposome tablet
[0105] The raw and auxiliary materials used are as follows:
[0106] Montelukast Sodium 5g
[0107] Hydrogenated Soy Lecithin 22g
[0108] Cholesterol 10g
[0109] Tween 80 4g
[0110] Mannitol 25g
[0111] Soy Sterol 2g
[0112] 60g pregelatinized starch
[0113] Microcrystalline Cellulose 30g
[0114] Low-substituted hydroxypropyl cellulose 10g
[0115] Hypromellose 2g
[0117] Preparation Process:
[0118] (1) 22g hydrogenated soybean lecithin, 10g cholesterol, 4g Tween 80 and 2g soybean sterol are dissolved in 600ml isopropanol to obtain lipid solution;
[0119] (2) Dissolve 5g montelukast sodium and 25g mannitol in 300ml water, slowly drop the above-mentioned lipid solution into it, and stir evenly with a magnetic stirrer at the same time, and the stirring time is 60min to obtain isopropanol-containing liposome suspension;
[0120] (3) Place the above-me...
Embodiment 2
[0126] The preparation of embodiment 2 montelukast sodium liposome capsules
[0127] The raw and auxiliary materials used are as follows:
[0128] Montelukast Sodium 5g
[0129] Hydrogenated Soy Lecithin 20g
[0130] Cholesterol 10g
[0131] Tween 80 4g
[0132] Mannitol 20g
[0133] Soy Sterol 3g
[0134] Starch 20g
[0135] Microcrystalline Cellulose 60g
[0136] Carboxymethyl Cellulose Calcium 10g
[0137] Povidone K30 4g
[0139] Preparation Process:
[0140] (1) 20g hydrogenated soybean lecithin, 10g cholesterol, 4g Tween 80 and 3g soybean sterol are dissolved in 300ml isopropanol to obtain lipid solution;
[0141] (2) Dissolve 5g montelukast sodium and 20g mannitol in 200ml water, slowly drop the above-mentioned lipid solution into it, and stir evenly with a magnetic stirrer at the same time, and the stirring time is 90min to obtain isopropanol-containing liposome suspension;
[0142] (3) Place the above-mentioned suspension in an ult...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com