Method for producing high-purity zinc sulfide and co-producing acetic acid and sodium chloride by using sodium hydrosulfite filter residues
A technology of sodium chloride and sodium hydrosulfite, applied in zinc sulfide, alkali metal chloride, organic chemistry, etc., can solve the problems of single use, inability to consume zinc powder waste residue, etc., achieve simple operation, less by-products, and be suitable for promotion Applied effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
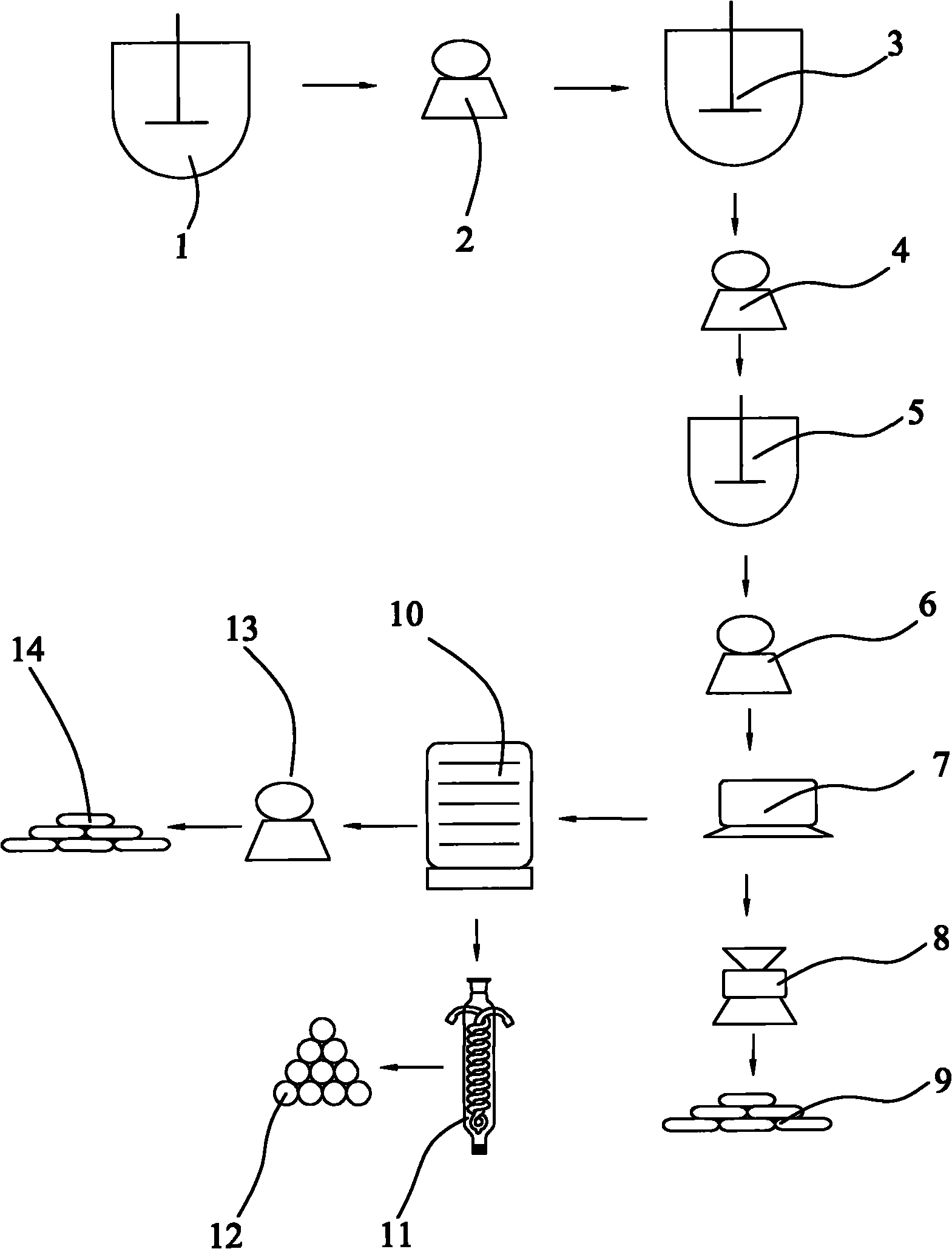
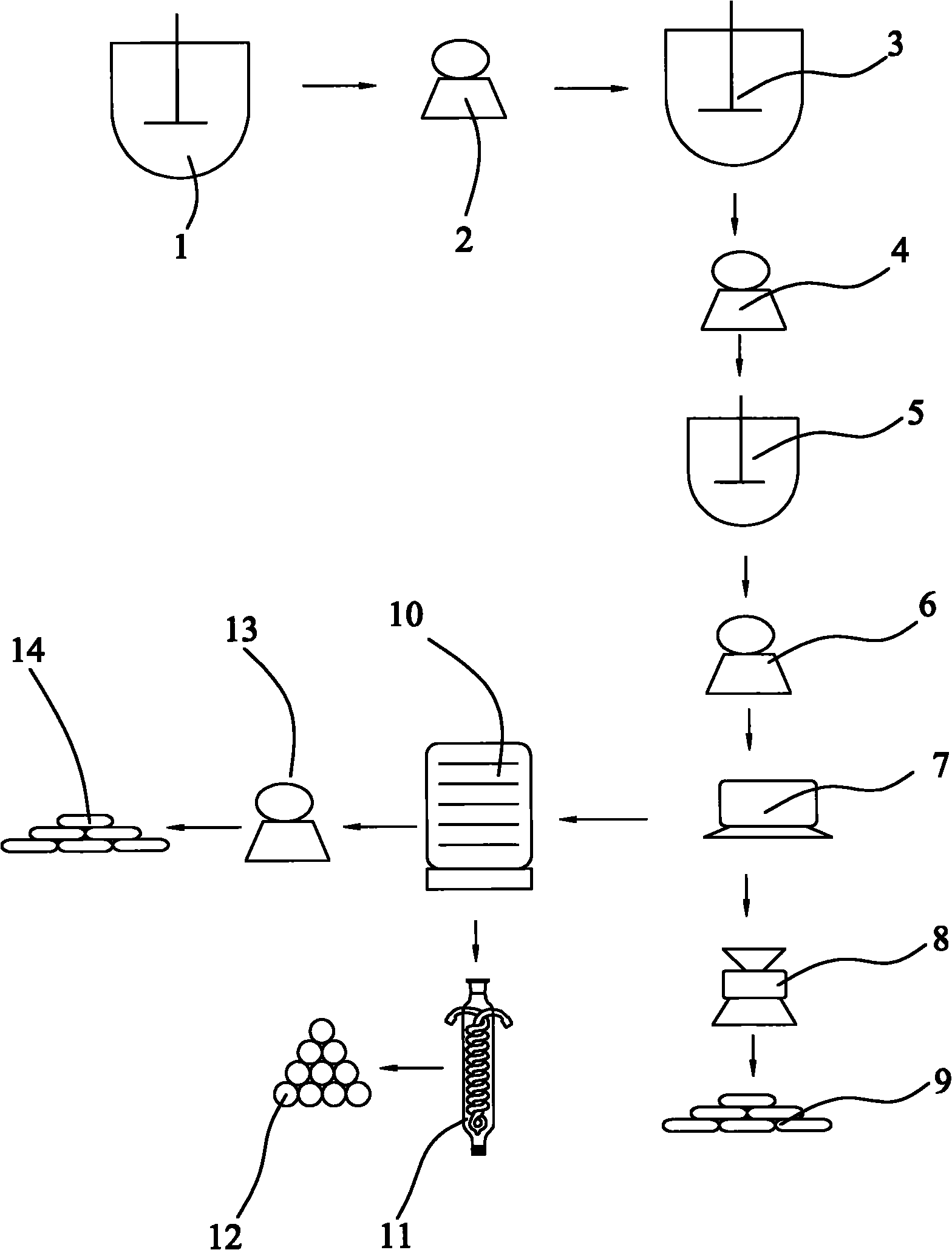
[0025] refer to figure 1 , a method of producing high-purity zinc sulfide co-production acetic acid and sodium chloride with sodium hydrosulfide filter residue, weighing 495kg sodium hydrosulfite, converting the content of zinc hydroxide in the filter residue of sodium hydrosulfite to be 297kg, and pulverizing the sodium hydrosulfite filter residue, configured into Suspension, configure dilute hydrochloric acid solution at the same time; Gained suspension and dilute hydrochloric acid solution are put into the first corrosion-resistant reactor (1) successively according to the mass ratio of zinc hydroxide and hydrogen chloride pure substance of 1: 0.74, and carry out under stirring reaction to obtain a zinc chloride-containing mixed solution; the gained zinc chloride-containing mixed solution is filtered through the first filter (2) to obtain a clear zinc chloride-containing filtrate; the clarified zinc chloride-containing filtrate is React with an appropriate amount of zinc hy...
Embodiment 2
[0027] refer to figure 1, a method of producing high-purity zinc sulfide co-production acetic acid and sodium chloride with sodium hydrosulfide filter residue, weighing 495kg sodium hydrosulfite, converting the content of zinc hydroxide in the filter residue of sodium hydrosulfite to be 297kg, and pulverizing the sodium hydrosulfite filter residue, configured into Suspension, configure dilute hydrochloric acid solution at the same time; Gained suspension and dilute hydrochloric acid solution are put into the first corrosion-resistant reactor (1) successively according to the mass ratio of zinc hydroxide and hydrogen chloride pure substance of 1: 0.94, and carry out under stirring reaction to obtain a zinc chloride-containing mixed solution; the gained zinc chloride-containing mixed solution is filtered through the first filter (2) to obtain a clear zinc chloride-containing filtrate; the clarified zinc chloride-containing filtrate is React with an appropriate amount of zinc hyd...
Embodiment 3
[0029] refer to figure 1 , a method of producing high-purity zinc sulfide co-production acetic acid and sodium chloride with sodium hydrosulfide filter residue, weighing 495kg sodium hydrosulfite, converting the content of zinc hydroxide in the filter residue of sodium hydrosulfite to be 297kg, and pulverizing the sodium hydrosulfite filter residue, configured into Suspension, configure dilute hydrochloric acid solution at the same time; Gained suspension and dilute hydrochloric acid solution are put into the first corrosion-resistant reactor (1) successively according to the mass ratio of zinc hydroxide and hydrogen chloride pure substance of 1: 0.54, and carry out under stirring reaction to obtain a zinc chloride-containing mixed solution; the gained zinc chloride-containing mixed solution is filtered through the first filter (2) to obtain a clear zinc chloride-containing filtrate; the clarified zinc chloride-containing filtrate is React with an appropriate amount of zinc hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com