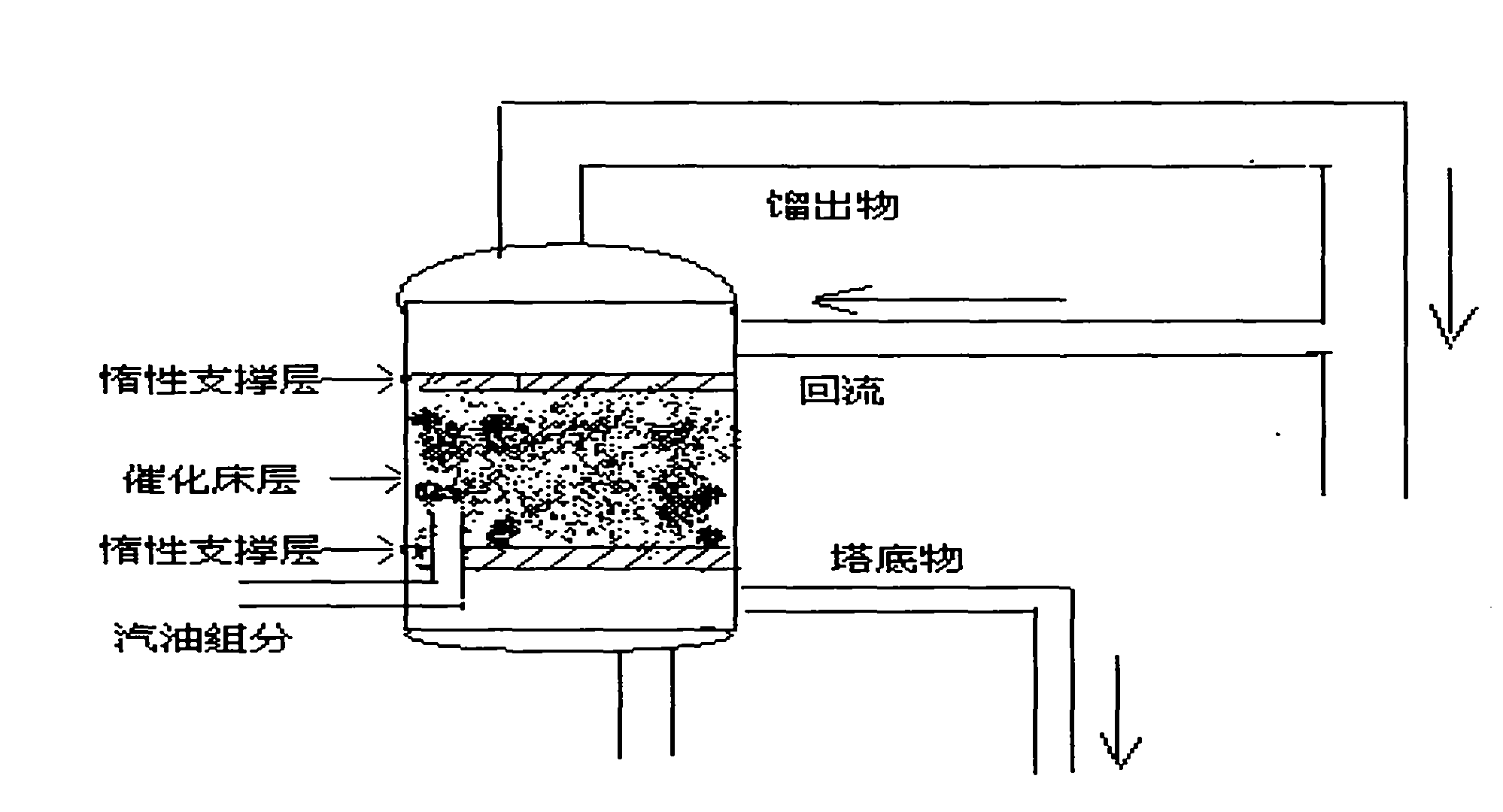
Method for removing thiophene sulfur-containing compound from petroleum
A compound, thiophene technology, applied in the field of petroleum oil alkylation desulfurization, can solve the problem of low selectivity, achieve high catalytic activity, low polymerization activity, and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] complexed with Fe 3+ Preparation of ionic cation exchange resin (NKC-9) catalyst:
[0047] Convert macroporous sulfonated styrene-diethylene copolymerized cation exchange resin into hydrogen-type resin, wash and dry with distilled water, take 30g into a 250ml three-hole flask, add 250ml, 1,2-dichloroethane, heat to 30°C Stir for 12 hours to fully swell the resin particles, then add 30g FeCl 3 , reacted under reflux for 16 hours, so that Fe 3+ A complex reaction occurs between the ions and the sulfonate groups in the resin skeleton, the resin is filtered out, washed with 1,2-dichloroethane until the filtrate is clear, the resin is taken out, dried in vacuum at 60°C for 24 hours, and set aside.
Embodiment 2
[0049] Alkylation desulfurization test:
[0050] In a stirred high-pressure reactor, add 150 grams of naphtha raw materials with a boiling point range of 62-210 ° C, complexed with Fe 3+ 0.48 g of strong acidic cation exchange resin catalysts of ions were reacted for 1 minute at a temperature of 180° C. and a pressure of 6 atmospheres. The reaction products were separated by gas chromatography based on differences in boiling points. The sulfur content in the distillate was detected by a sulfur chemiluminescence detector, and the sulfur content of the gasoline component with a boiling point of 62-177° C. was 18.5 ppm.
Embodiment 3
[0052] Alkylation desulfurization test:
[0053] In a stirred autoclave, add 120 g of simulated gasoline components composed of octane, benzothiophene, and 2-methylbutene, the sulfur content of which is 850 ppm, and then add 0.56 g of complexed Fe 3+ The strongly acidic cation exchange resin of ions was reacted for 2 minutes at a stirring speed of 500 rpm, 204° C., and 10 atmospheric pressure. The reaction products were separated by gas chromatography based on differences in boiling points, and the sulfur content in the fractions was determined with a sulfur chemiluminescence detector. The sulfur content of components within the boiling range of gasoline at the boiling point is reduced to 15ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com