Medicinal composition for preventing and treating atherosclerosis
A technology of atherosclerosis and composition, applied in the field of pharmaceutical composition for preventing and treating atherosclerosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Extraction, separation and purification of tanshinone II-A
[0028] Dried Salvia miltiorrhiza 1 kg, extracted with 95% ethanol three times (1000 ml each time), 24 hours each time, concentrated the extract to 500 ml under reduced pressure, then added 500 ml water, and divided it four times with 1000 ml chloroform Extraction, concentration of the extract under reduced pressure, separation by column chromatography, the silica gel is 100-200 mesh, the eluent is a mixed solution of petroleum ether / ethyl acetate containing 1%-10% ethyl acetate, and gradient elution is carried out. About 1.8 g of tanshinone II-A with a purity of 98% can be obtained.
Embodiment 2
[0029] Example 2 Effect of combined administration of Tanshinone II-A and atorvastatin on blood lipids in ApoE knockout mice
[0030] 5-week-old male ApoE knockout mice (ApoE - / - ) was purchased from the Department of Experimental Animals, Peking University Health Science Center (introduced from the Jackson Laboratory of the United States, animal certificate number: SCXK (Beijing) 2006-0008). C57BL / 6J male mice of the same strain and the same week of age were purchased from the Experimental Animal Center of Southern Medical University (5 weeks old, SPF grade, animal certificate number: SCXK (Guangdong) 2006-0015). After the experimental animals were bought back, they were kept in the SPF environment of the Experimental Center of the School of Public Health, Sun Yat-sen University (temperature 25±0.5 degrees, humidity 60%-70%, light 12h / dark 12h), and adaptive feeding for 1 week, and the next week (6 weeks) Start feeding high-fat feed (basic feed + 10% lard + 1.25% cholesterol...
Embodiment 3
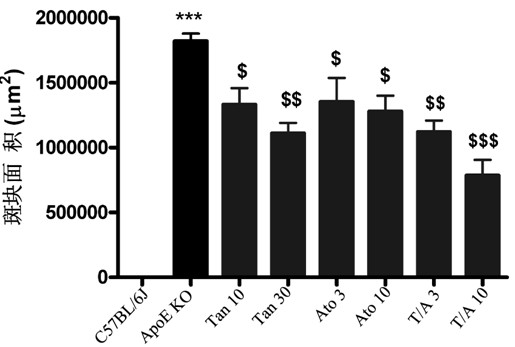
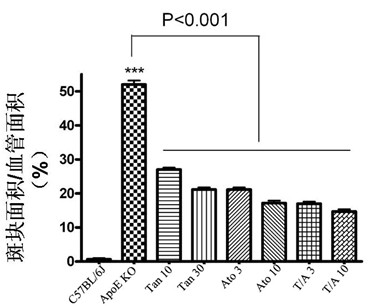
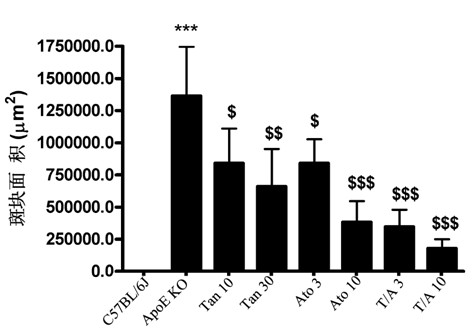
[0035] Example 3 Effect of combined administration of Tanshinone II-A and atorvastatin on the area of aortic sinus plaque in ApoE knockout mice
[0036] The experimental grouping is the same as in Example 2. The perfused heart was removed, rinsed with PBS to remove residual blood, and cut with a blade parallel to the line where the left and right atrial appendages were located or perpendicular to the aortic root, leaving the upper half of the heart tissue (with a 1 mm aortic root). arteries), coated in a mold containing OCT (Tissue-Tek), immediately frozen in liquid nitrogen and stored in a -80°C refrigerator. Frozen slices were frozen on a Leica cryostat, and the slices were continuously sliced until the three valves of the aortic sinus completely appeared, and the slices were collected with a thickness of 8 μm, and a slice was attached at an interval of 50 μm. ) lay out 6 slices. After cutting the slices, store the slices at -80°C. Sections were stained with Oil Red O...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com