Phosphorescent iridium complex and electroluminescence device thereof
A technology of phosphorescent iridium complexes and complexes, which is applied in electric solid devices, electrical components, semiconductor devices, etc., can solve problems such as insufficient thermal stability, unsatisfactory energy level structure, triplet-triplet quenching, etc. Achieve the effect of reducing self-quenching, improving electrical properties, and reducing direct effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
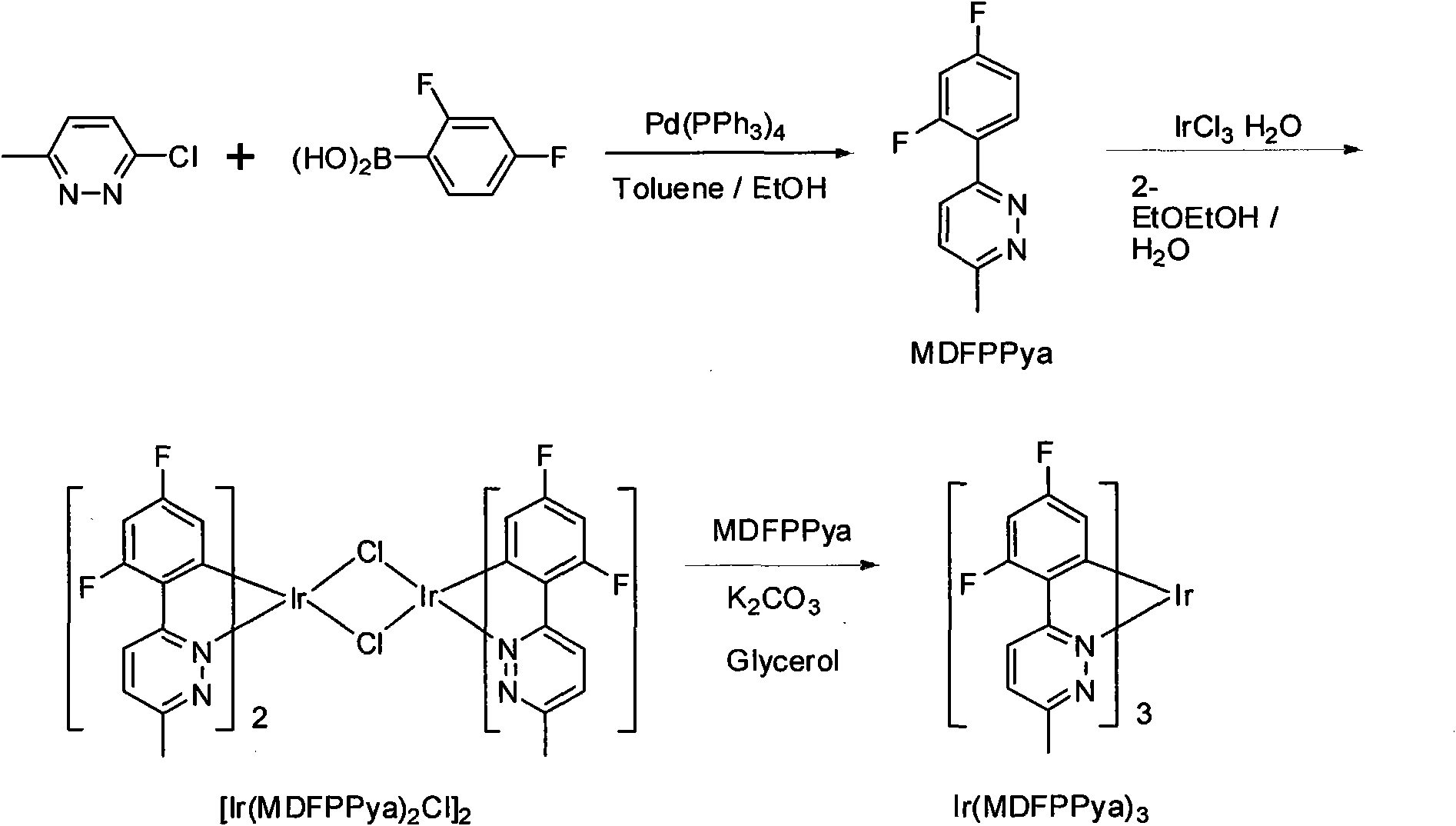
[0032] Example 1. Synthesis of 3-methyl-6-di2',4'-(fluorophenyl)pyridazine [3-methyl-6-(2,4-difluoro-phenyl)pyridazine, referred to as MDFPPya]. Take 250mg of 3-methyl-6-chloropyridazine, 463mg of 2,4-difluorophenylboronic acid and 67mg of tetraphenylphosphopalladium in a two-necked flask, add 12ml of toluene, 2ml of ethanol and 2.1ml of 2M K 2 CO 3 solution. At a temperature of 100°C, reflux for 24h. Stand to cool, quench with dichloromethane, and then use anhydrous magnesium sulfate to remove water. The solvent was evaporated under reduced pressure, and the ligand MDFPPya (79%) was obtained by silica gel chromatography.
Embodiment 2
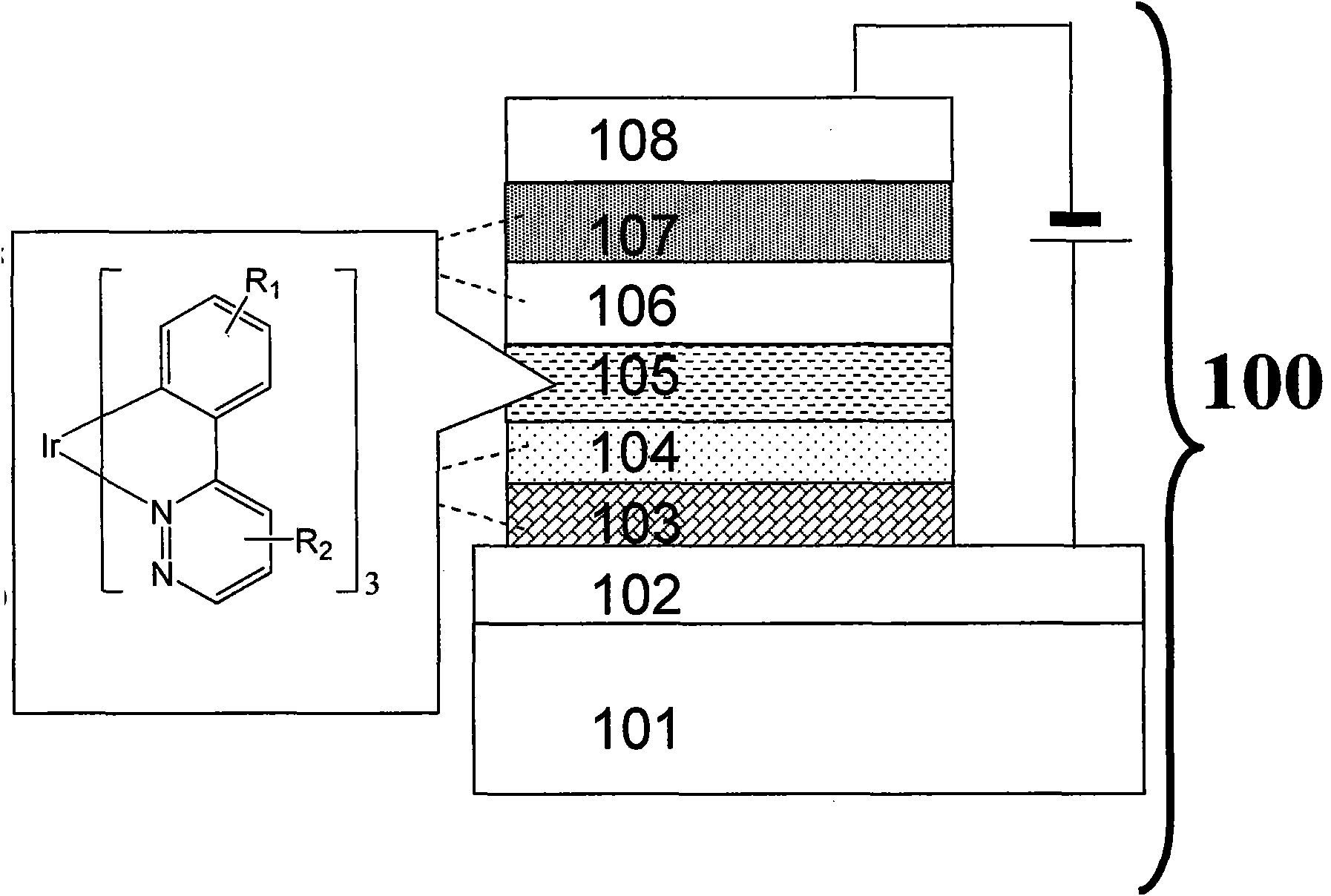
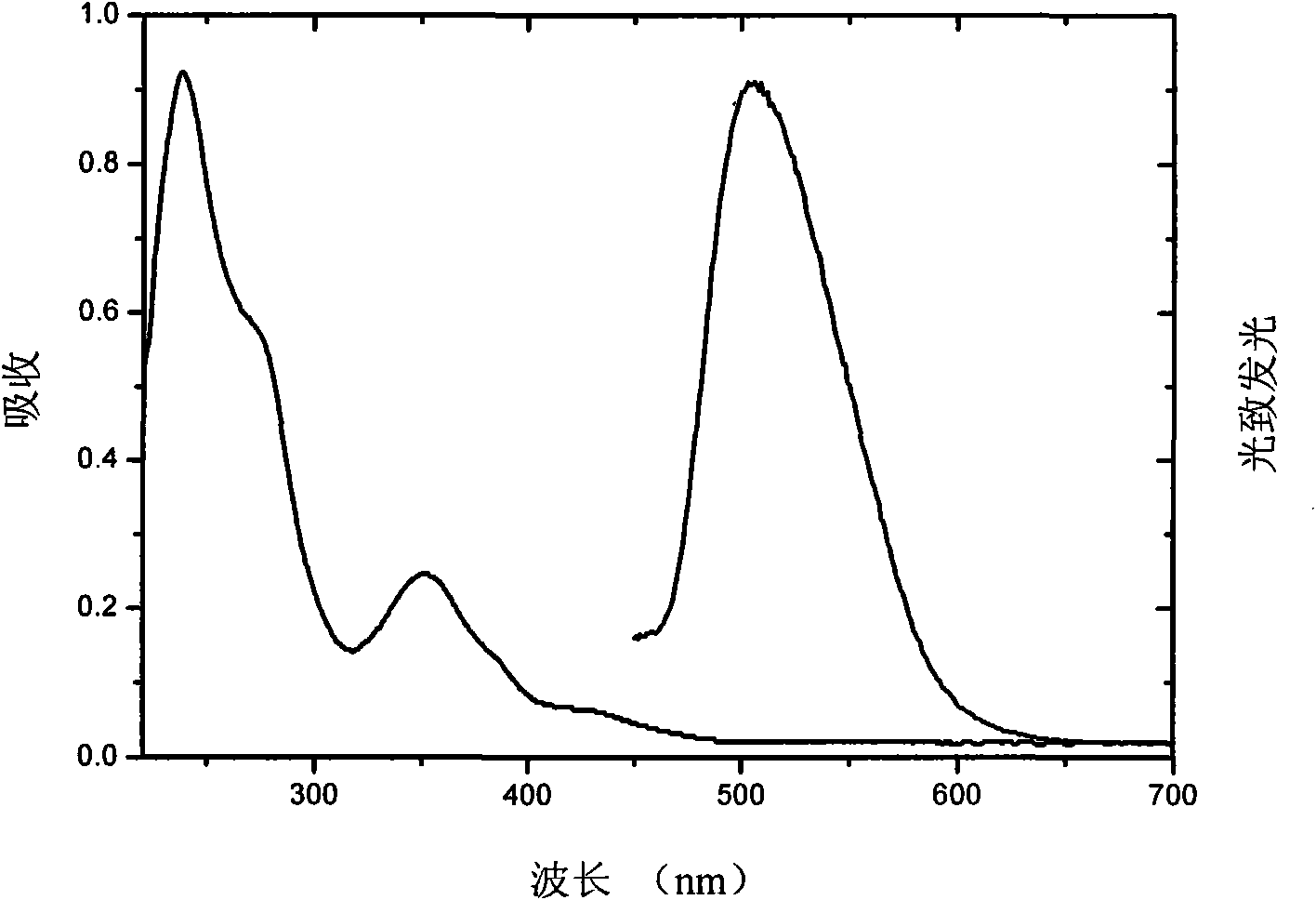
[0033] Example 2, three (3-methyl-6-two (2 ', 4'-fluorophenyl) pyridazine) iridium [tris [3- (2, 4-difluoro-phenyl) -6-methylpyridazinato-N 1 , C 2 ]iridium, abbreviated as Ir(MDFPPya) 3 ]Synthesis. Take the ligand 3-methyl-6-bis(2',4'-fluorophenyl)pyridazine (MDFPPya) 163mg and 100mg IrCl 3 ·H 2 O In a two-necked flask, add 6ml of 2-ethoxyethanol and 2ml of deionized water. At a temperature of 110°C, reflux for 12h. Let stand to cool, add 5ml deionized water, and filter. After vacuum drying at 60°C for 4 hours, 151mg (75%) of chlorine-bridged dimer was obtained. Then dimer, 62mg MDFPPya and 104mg K 2 CO 3 Add to the two-necked bottle, add 5ml glycerin. At a temperature of 185°C, react for 12h. Standing for cooling, quenching with dichloromethane and distilling under reduced pressure to remove solvent, silica gel chromatography to obtain the final product Ir(MDFPPya) 3 135mg (71%). Ir(MDFPPya) 3 The NMR spectrum is: 1 HNMR (TMS is internal standard, solvent CDCl ...
Embodiment 3
[0034] Example 3, three-(3,6-bis(4'-fluorophenyl) pyridazine) iridium [tris[3,6-bis(4-fluorophenyl)pyri-dazinato-N 1 , C 2 ]iridium, referred to as Ir(BFPPya) 3 ]Synthesis. in N 2 Under protection, 378.0mg 3,6-bis(4'-fluorophenyl)pyridazine (BFPPya) and 172.4mg tris(acetylacetonato)iridium(III)[Ir(acac) 3 ] into 5ml glycerin. Raise the temperature to 50°C, and pump air for 4 hours with a vacuum pump. Then the temperature was raised to 190°C and refluxed for 12h. After a large amount of yellow solids were precipitated, stop the reaction, cool to room temperature, extract with dichloromethane, distill off the solvent under reduced pressure, filter, wash with a small amount of methanol, and dry in vacuo to obtain 232 mg of orange-yellow solid powder (crude yield 67.6%) ). Then it was separated by silica gel column chromatography using dichloromethane as the eluent to obtain an orange-yellow solid powder. Ir(BFPPya) 3 The NMR spectrum is: 1 HNMR (TMS is internal standard...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com