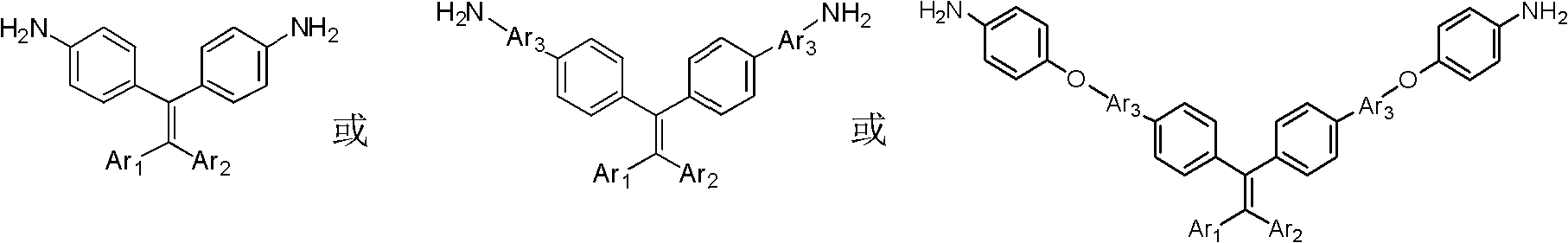
Novel functional diamine monomer with large conjugate structure, and preparation method and application thereof
A technology of diamine monomer and conjugated structure, which is applied in the field of material science, can solve the problems of single species and performance to be further improved, and achieve the effects of high yield, easy purification and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
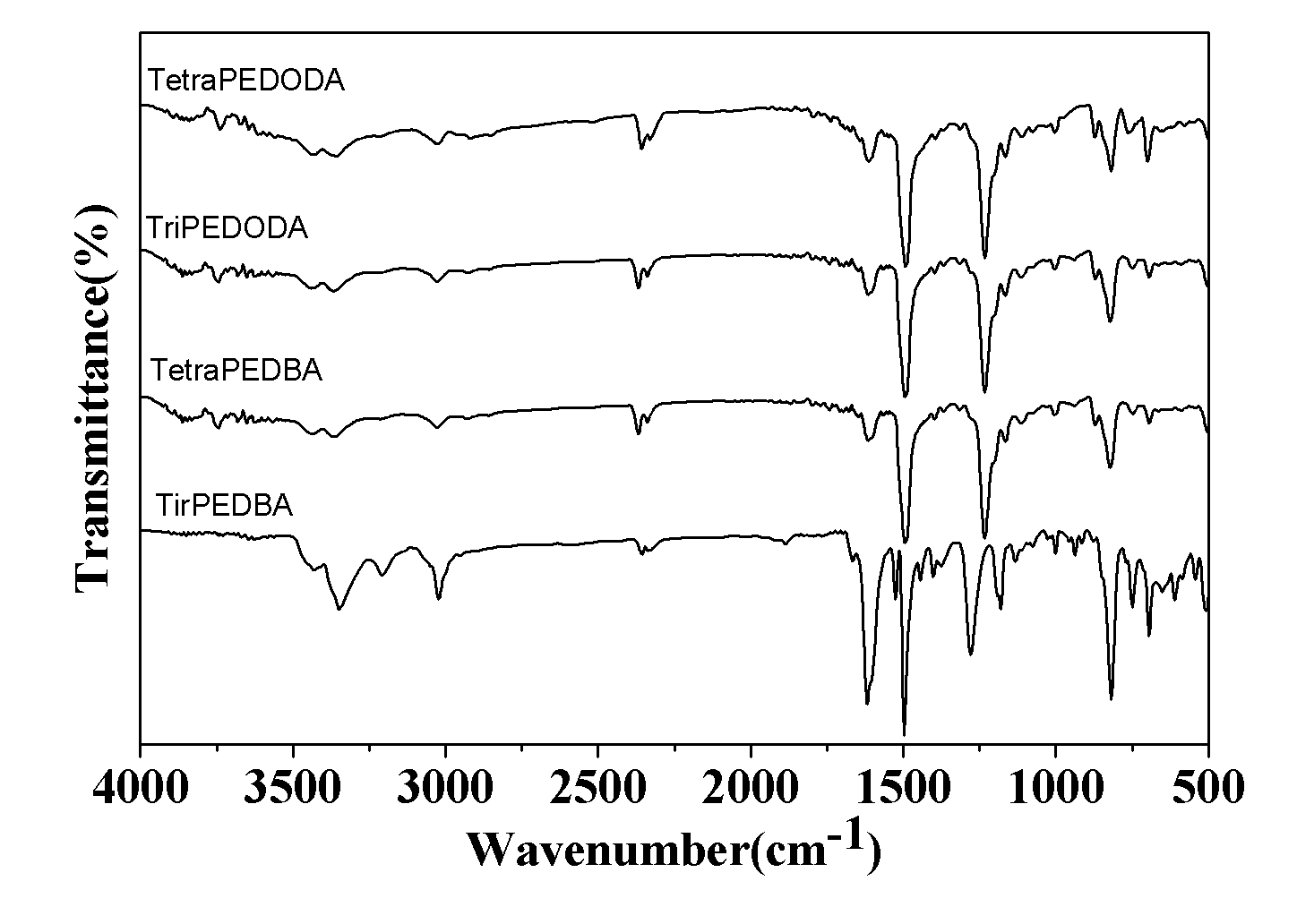
Image
Examples
Embodiment 1
[0021] 4', 4 "-(2-phenylethene-1, 1-diyl) dibiphenyl-4-amine (TriPEDBA) synthesis:
[0022]
[0023] (1) Synthetic intermediate 4,4'-(2-phenylethene-1,1-diyl)bis(bromobenzene)(TriPEDBr):
[0024] Add 11.6ml (0.1mol) of benzyl chloride and 52.2ml (0.3mol) of triethyl phosphite into a 500ml three-neck flask, magnetically stir and pass argon, heat and reflux in an oil bath at 150°C for 24h, cool to room temperature, and pour Add 300ml tetrahydrofuran (THF) to the three-necked flask, then add 16.82g potassium tert-butoxide (t-BuOK 0.15mol) and 27.2g dibromobenzophenone (bis(4-bromophenyl)methanone 0.08mol), stir magnetically for 24h, and react After stopping, the reaction solution was poured into water for extraction, and a large amount of white precipitates precipitated out, filtered through a funnel, collected the precipitates, washed with ethanol three times, and dried in vacuum at 60°C for 10 hours to obtain 31.5 g of the white intermediate product TPEDBr. The rate is 95%....
Embodiment 2
[0029] 4 ', 4 "-(2,2-diphenylethene-1,1-diyl) dibiphenyl-4-amine (TetraPEDBA) synthesis:
[0030]
[0031] (1) Synthetic intermediate 4,4'-(2,2-diphenylethene-1,1-diyl)bis(bromobenzene)(TetraPEDBr):
[0032] Add 6.18ml (0.0368mol) of diphenylmethane (diphenylmethane) and 100ml of tetrahydrofuran (THF) into a 500ml three-necked flask, magnetically stir and pass argon, and after 40min of ice-salt bath, add 21.7ml (0.04784mol ) n-butyllithium solution, after continuing the ice-salt bath for 40min, add 10g of dibromobenzophenone (bis(4-bromophenyl)methanone 0.0217mol), after reacting at room temperature for 12h, add 100ml of saturated ammonium chloride (NH 4 Cl) solution, after stirring for 10min, the reaction solution was poured into a separatory funnel, the upper layer was taken, and the water in the solution was adsorbed with 150g of anhydrous sodium sulfate, added to a three-necked flask after suction filtration, and 100ml of toluene was used as a solvent, magnetically stir...
Embodiment 3
[0037] 4,4′-(4′,4″-(2-phenylethene-1,1-diyl)bis(biphenyl-4′,4-diyl))bis(oxy)dibenzonamine (TriPEDOBA) synthesis:
[0038]
[0039] (1) Synthetic intermediate 4′, 4″-(2-phenylethene-1, 1-diyl)dibiphenyl-4-ol (TriPEDO):
[0040] First, TriPEDBr was synthesized according to Example 1. Weigh 6.0318g (0.015mol) of TriPEDBr and 4.1379g (0.03mol) of p-hydroxyphenylboronic acid (4-hydroxyphenylboronic acid) into a 500ml three-necked flask, add 100ml of tetrahydrofuran, then add 45ml of 2mol / L potassium carbonate solution, and stir magnetically And pass argon, heat the oil bath to 70°C, add 0.05g tetrakistriphenylphosphine palladium, reflux reaction for 24 hours, pour the reaction solution into water for extraction, a large amount of white precipitates precipitate out, filter with a funnel, collect the precipitates , washed 3 times with ethanol, dried to obtain off-white product 5.4g, productive rate is 84%, and its structural formula is as follows:
[0041]
[0042] (2) Synthe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com