Method for synthesizing diindolylmethane derivative
A technology of diindolylmethane and a synthesis method, which is applied in the field of compound synthesis, can solve the problems of large solvent pollution, complicated treatment procedures, long reaction time and the like, and achieves that the synthesis process is simple and easy to operate, shortens the process time, and has good product quality. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Take 10g of Hβ molecular sieve, add 40mL of oxalic acid aqueous solution with a concentration of 1.0mol / L, stir at 50°C for 1h, filter, wash, dry in an oven at 120°C, and roast in a muffle furnace at 550°C for 4h to obtain oxalic acid-modified Hβ molecular sieve catalyst.
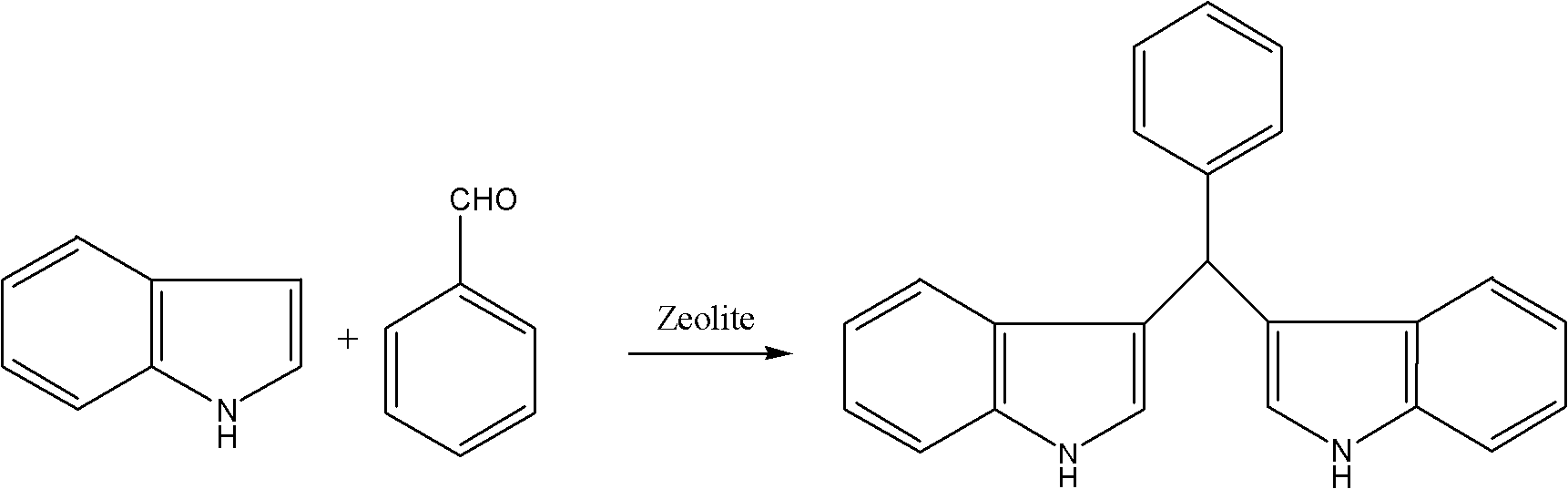
[0021] Take 5 g of oxalic acid-modified Hβ molecular sieves and put them into a fixed-bed reactor, dissolve 1 mol of indole and 0.25 mol of benzaldehyde in 500 mL of ethanol solution, set the reaction temperature at 80 ° C, and dissolve the ethanol solution containing indole and benzaldehyde Pass it into a fixed-bed reactor at a rate of 0.5mL / min to react continuously to generate phenyldiindolylmethane. Concentrate 20 mL of the reaction solution and add 10 mL of water to precipitate the product. After filtration and drying, phenyldiindolylmethane is obtained with a yield of 95.2%.
[0022] Its specific reaction formula is:
[0023]
[0024] m.p.: 93-95°C; 1 H NMR (600MHz, CDCl 3 ): δ7.57(s, 2H...
Embodiment 2
[0026] Take 10g of Hβ molecular sieve, add 40mL of phosphoric acid aqueous solution with a concentration of 1.2mol / L, stir at 55°C for 1.5h, filter, wash, dry in an oven at 120°C, and roast in a muffle furnace at 500°C for 3h to obtain phosphoric acid-modified Hβ molecular sieve catalyst.
[0027] Take 5g of phosphoric acid-modified Hβ molecular sieve and put it into a fixed-bed reactor, dissolve 1.25mol indole and 0.25mol benzaldehyde in 500mL ethanol solution, set the reaction temperature to 100°C, and dissolve the indole and benzaldehyde containing The ethanol solution is fed into the fixed-bed reactor at a rate of 1mL / min to react continuously to generate phenyldiindolylmethane; after 20mL of the reaction solution is concentrated, 10mL of water is added to precipitate the product, which is filtered and dried to obtain phenyldiindolylmethane. The yield was 93.6%.
[0028] m.p.: 93-95°C; 1 H NMR (600MHz, CDCl 3 ): δ7.57(s, 2H), 7.33(d, J=8.0Hz, 2H), 7.28(d, J=7.5Hz, 2H), ...
Embodiment 3
[0030] Take 10g of Hβ molecular sieve, add 40mL of citric acid aqueous solution with a concentration of 0.8mol / L, stir at 40°C for 0.5h, filter, wash, dry in an oven at 120°C, and roast in a muffle furnace at 600°C for 2h to obtain citric acid Modified Hβ molecular sieve catalyst.
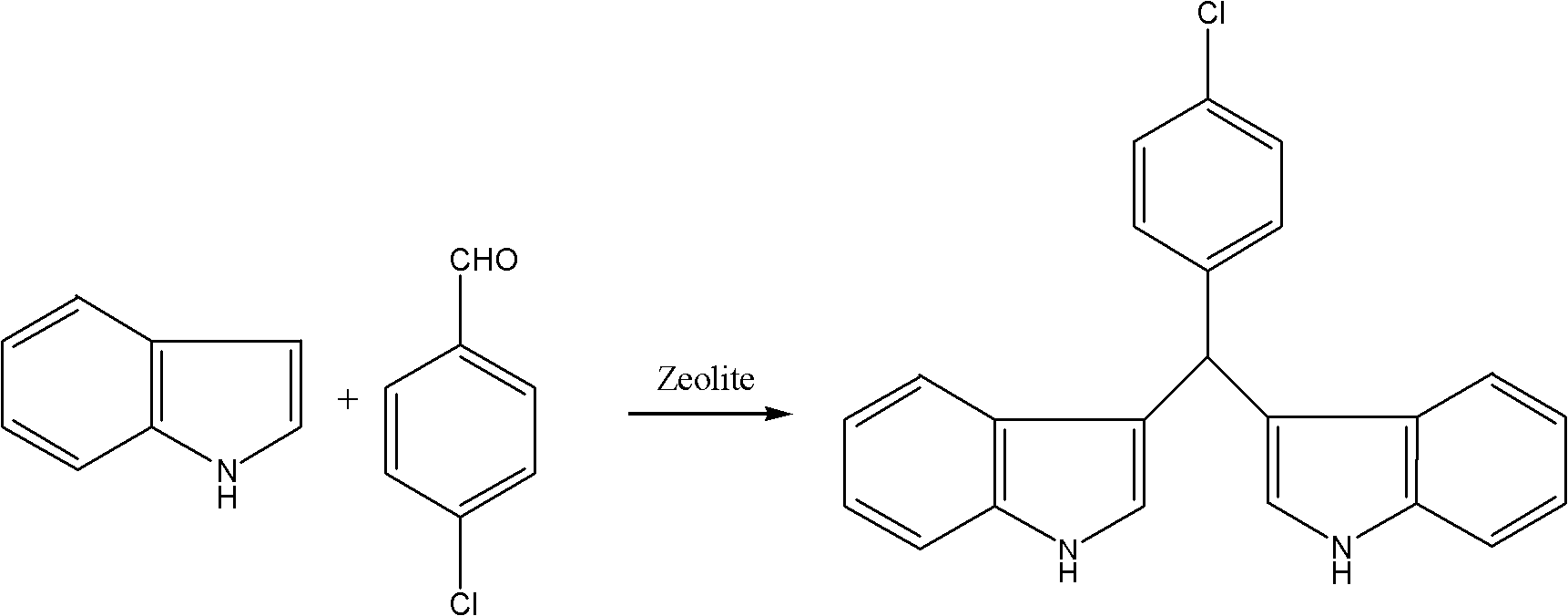
[0031] Take 5g of citric acid-modified Hβ molecular sieve and put it into a fixed-bed reactor, dissolve 1.2mol of indole and 0.25mol of 4-chlorobenzaldehyde in 500mL of ethanol solution, set the reaction temperature to 100°C, and mix the indole and The ethanolic solution of 4-chlorobenzaldehyde is passed into the fixed-bed reactor with the speed of 0.5mL / min and reacts to generate 4-chlorophenyldiindolylmethane continuously; After getting 20mL reaction solution to concentrate, add 10mL water and can separate out product, through After filtration and drying, 4-chlorophenyldiindolylmethane was obtained with a yield of 94.3%.
[0032]
[0033] m.p.: 76-78°C; 1 H NMR (600MHz, CDCl 3 ): δ7.67(s, 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com