Bone growth factor controlled release type bone repairing material as well as preparation method and applications thereof
A bone growth factor and repair material technology, applied in the field of bone growth factor controlled-release bone repair materials and its preparation, can solve the conditions of poor long-term sustained release of bone growth factor and difficulty in providing synergistic effects of multiple growth factors, etc. To achieve the effect of exerting osteoinductive effect, promoting bone tissue regeneration and functional reconstruction, and meeting the requirements of bone defect repair
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
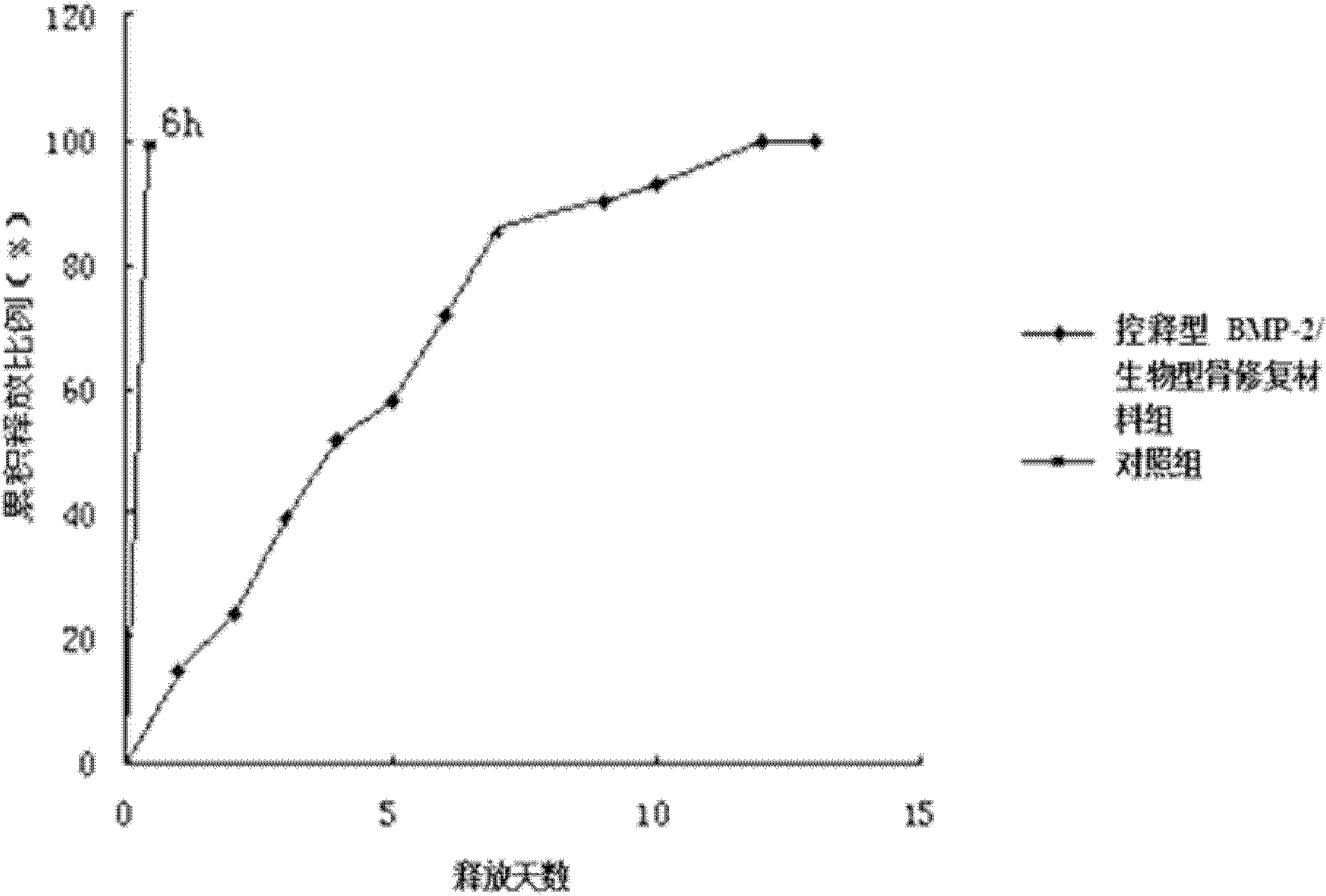
[0036] Example 1 Controlled release BMP-2 / biological bone repair material
[0037] (1) Weigh 1.0 g of chitosan dissolved in 0.5% (v / v) acetic acid solution, prepare a chitosan solution with a concentration of 0.1% by mass and volume, and then stir (rotate at 800r / min), press The mass ratio of bone morphogenetic protein-2 (BMP-2) to chitosan is 0.05:1 and BMP-2 is added and mixed evenly;
[0038] (2) Weigh 1.0g of sodium hyaluronate, dissolve it in deionized water, and prepare a solution with a concentration of 0.1% by weight;
[0039] (3) Weigh 0.5g of the biological porous bone repair material (sample from Guangdong Guanhao Biotechnology Co., Ltd.) and immerse it in the final solution prepared in step (1) under negative pressure (0.01MPa), keep it for 1 hour, and take it out to dry ;
[0040] (4) Immerse the porous bone repair material processed in step (3) into the final solution made in step (2) under negative pressure (0.1MPa), keep it for 1 hour, take it out to dry, and then ste...
Embodiment 2
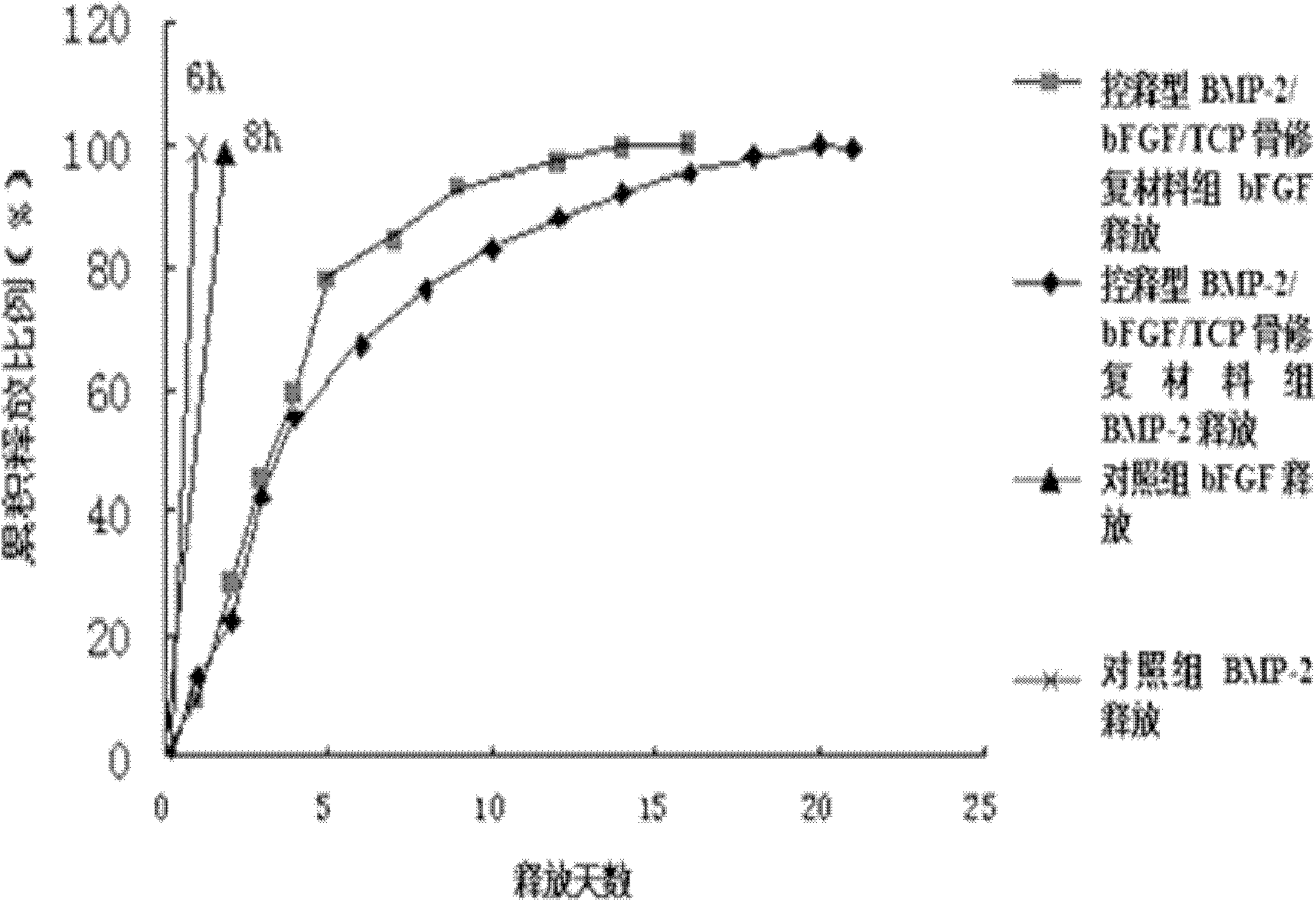
[0042] Example 2 Controlled-release BMP-2 / bFGF / TCP bone repair material
[0043] (1) Weigh 1.0g of chitosan dissolved in 1.0% (v / v) acetic acid solution, prepare a solution with a concentration of 0.05% by mass and volume, and then press BMP-2 under stirring (speed 3000r / min) Add BMP-2 and bFGF in a ratio of 0.1:0.001:1 with the mass ratio of bFGF to chitosan, and mix well;
[0044] (2) Weigh 1.0 g of carboxymethyl chitosan and dissolve it in deionized water to prepare a solution with a concentration of 0.05% by mass and volume;
[0045] (3) Weigh 0.5g calcium-phosphorus bioceramic porous bone repair material TCP (Wuhan Warwick Biomaterials Engineering Co., Ltd. β-TCP artificial bone, trade name: Biomet artificial bone) immersion step (1) under negative pressure (0.1MPa) ) In the prepared solution, keep for 2 hours, take out and dry;
[0046] (4) Immerse the porous bone repair material processed in step (3) into the solution made in step (2) under negative pressure (0.01MPa), keep fo...
Embodiment 3
[0048] Example 3: Controlled release BMP-2 / bFGF / biological bone repair material
[0049] (1) Weigh 1.0g of chitosan dissolved in 1.0% (v / v) acetic acid solution, prepare a solution with a concentration of 0.05% by mass and volume, and then press BMP-2 under stirring (speed 2000r / min) Add BMP-2 with a mass ratio of 0.1:1 to chitosan and mix well;
[0050] (2) Weigh 1.0g of hyaluronic acid and dissolve it in deionized water to prepare a solution with a concentration of 0.05% by mass and volume. Then, under stirring, add bFGF in a ratio of 0.001:1 by mass of bFGF to hyaluronic acid. ,well mixed;
[0051] (3) Weigh 0.5g of the biological porous bone repair material and immerse it in the solution made in step (1) under negative pressure (0.1MPa), keep it for 1 hour, and take it out to dry;
[0052] (4) Immerse the porous bone repair material processed in step (3) into the solution made in step (2) under negative pressure (0.1MPa), keep it for 1 hour, take it out to dry, and then sterilize...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of deacetylation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com