3,5-diaminopyridine and preparation method of 3,5-dimethoxycarboxylaminopyridine
A technology of diaminopyridine and pyridine dicarboxylic acid, applied in 3 fields, can solve the problems of limited application, high price, difficult to obtain, etc., and achieves the effects of expanding the scope of application, convenient removal, and simple processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
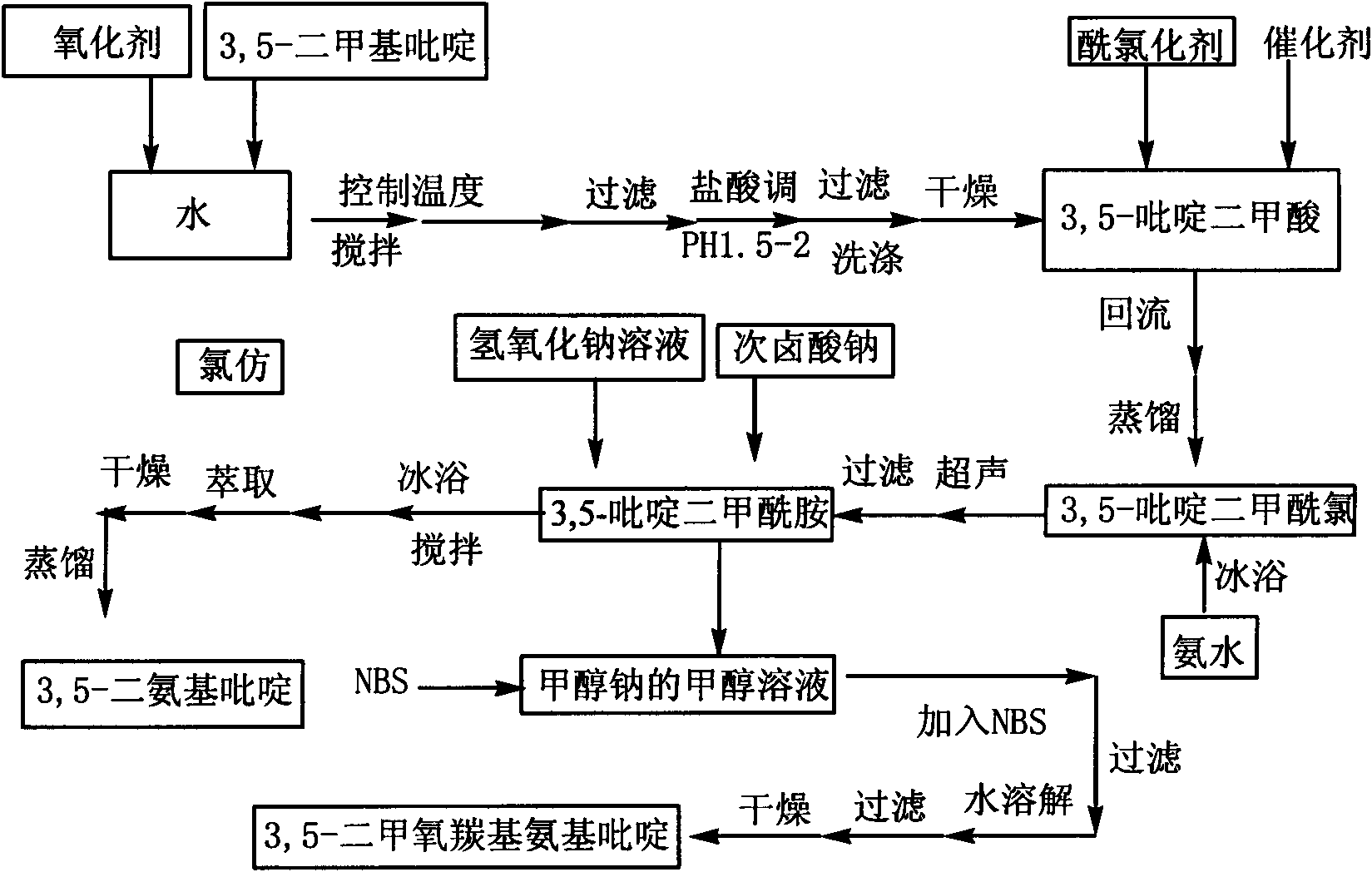
[0027] combine figure 1 , the preparation method of 3,5-diaminopyridine of the present invention, the steps are as follows:
[0028] In the first step, 3,5-lutidine is oxidized to obtain 3,5-pyridinedicarboxylic acid; the oxidizing agent for the oxidation reaction includes potassium permanganate, potassium dichromate, chromium oxide or tin dioxide; the oxidation reaction The temperature is 50-90°C; the molar ratio of oxidation reaction oxidant to 3,5-lutidine is 2:1-5.5:1.
[0029] In the second step, 3,5-pyridinedicarboxylic acid is prepared by acid chlorination reaction to obtain 3,5-pyridine dicarboxylic acid chloride; acid chloride reaction acid chloride reagent is selected from thionyl chloride, phosphorus trichloride or oxalyl chloride; acid chloride reaction temperature 56~65℃; the acid chloride reagent is used as both the reaction reagent and the solvent in the acid chloride reaction, and the acid chloride reagent is in excess of 3,5-pyridine dicarboxylic acid.
[00...
Embodiment 1
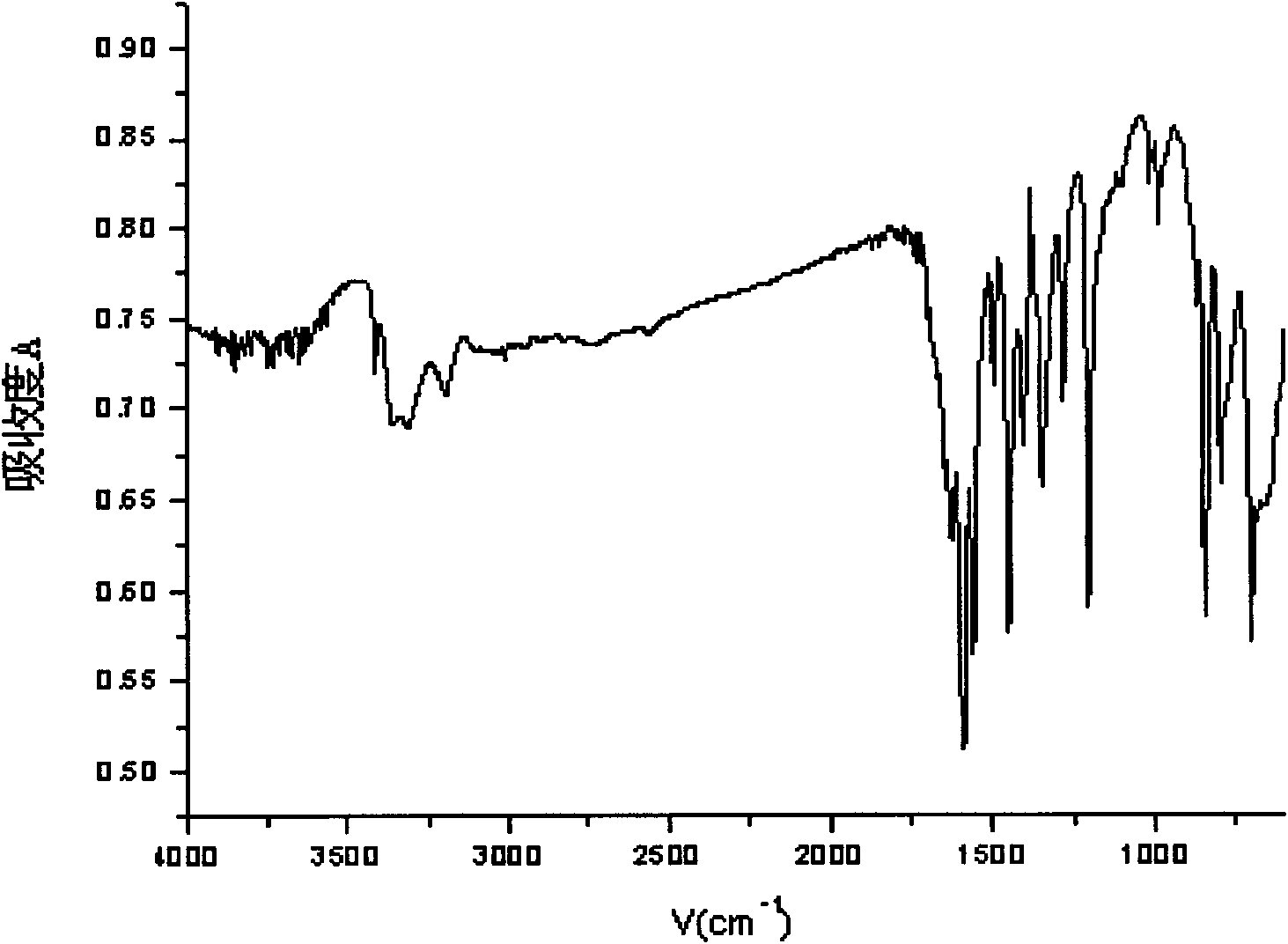
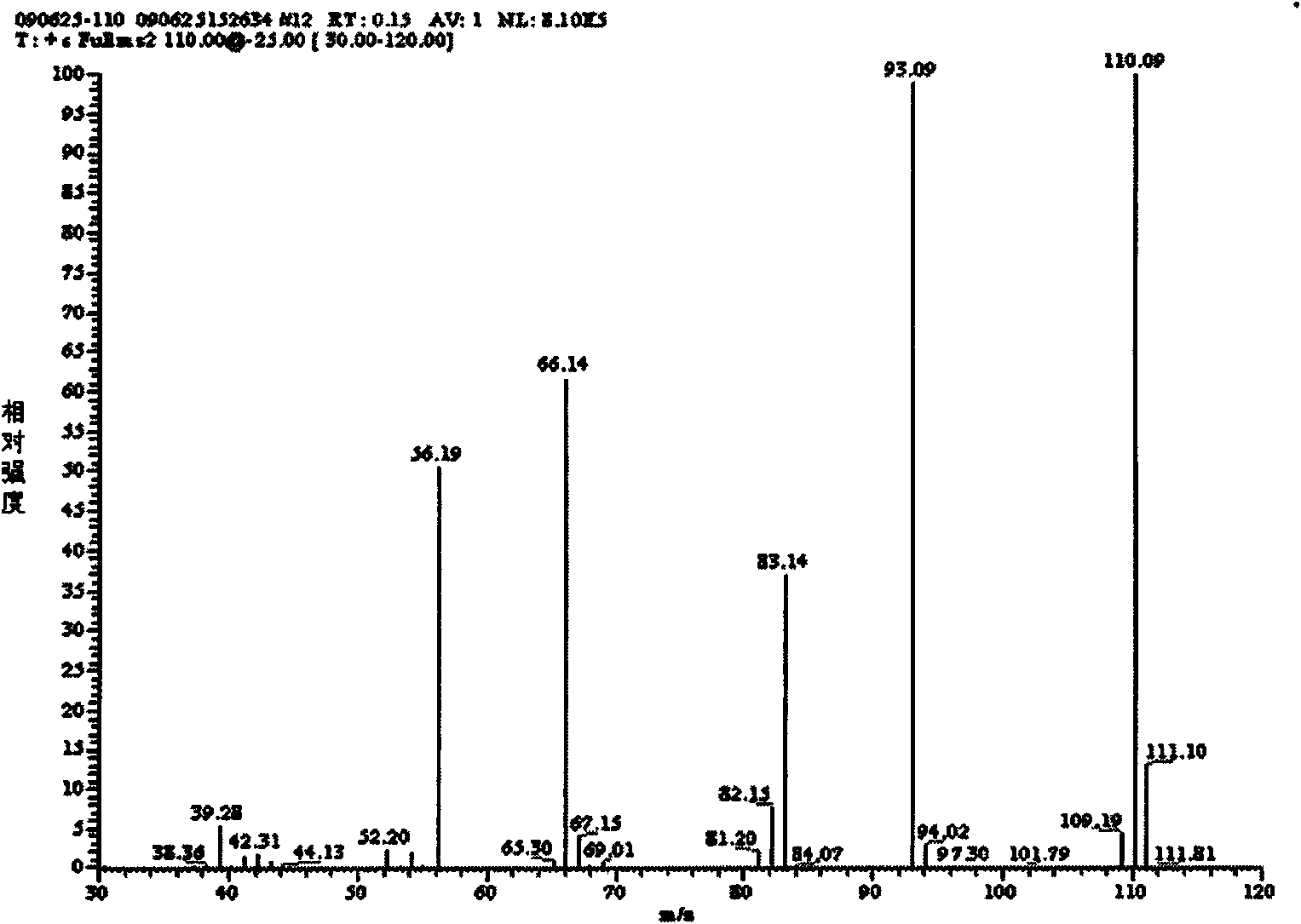
[0038] Example 1: Add 5ml of 3,5-lutidine (specific gravity: 0.99) into a three-necked flask filled with 75ml of water, stir and heat up to 40°C, add 29g of potassium permanganate in batches, complete the addition, and react at a constant temperature 2h. Cool to room temperature, filter with suction, adjust the pH of the filtrate to 1.5 to 2.0 with hydrochloric acid, precipitate a white precipitate, filter with suction, wash with 100ml of water, and dry the filter cake to obtain 5g of white powder of 3,5-pyridinedicarboxylic acid with a yield of 65%. m.p.324~326℃; 1 H NMR (DMSO-d 6 , 300MHz); δ: 8.65 (s, 1H, Ar-H), 9.25 (s, 2H, Ar-H), 13.71 (brs, 2H, -COOH); IR, (KBr) v: 3088 (C-H), 2914(O-H), 1720(C=O), 1600(C=C), 1163(C-O), 775(C-H)cm -1 ; Mass Spectrum: MS (ESI) m / z: 165.97 (30), MS / MS: 121.97 (100); it can be determined that the generated substance is 3,5-pyridinedicarboxylic acid.
Embodiment 2
[0039] Example 2: Add 5ml of 3,5-lutidine (specific gravity: 0.99) into a three-necked flask filled with 75ml of water, stir and heat up to 60°C, add 38g of potassium permanganate in batches, complete the addition, and react at a constant temperature 1h. Cool to room temperature, filter with suction, adjust the pH of the filtrate to 1.5-2.0 with hydrochloric acid, precipitate a white precipitate, filter with suction, wash with 100ml of water, and dry the filter cake to obtain 6.16 g of white powder of 3,5-pyridinedicarboxylic acid, yield 79.7 %.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com