Liparis nervosa lectin gene and protein as well as application thereof
A technology of lectin and lectin, which is applied in the field of lectin, can solve the problems that have not been reported, and achieve the effect of expanding the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: Preparation of lectin protein
[0016] In the present embodiment, the preparation method of the lectin protein of sageria spp. is as follows
[0017] 1. Crude product preparation
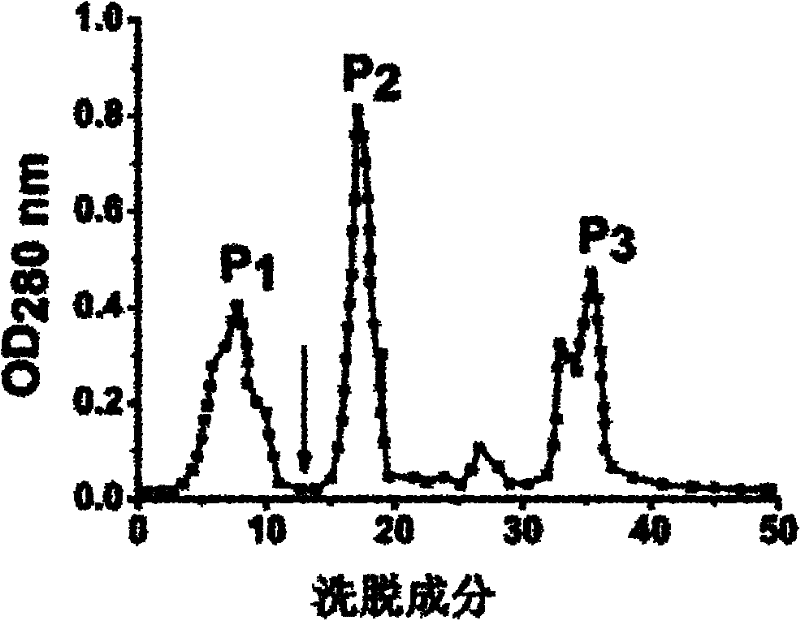
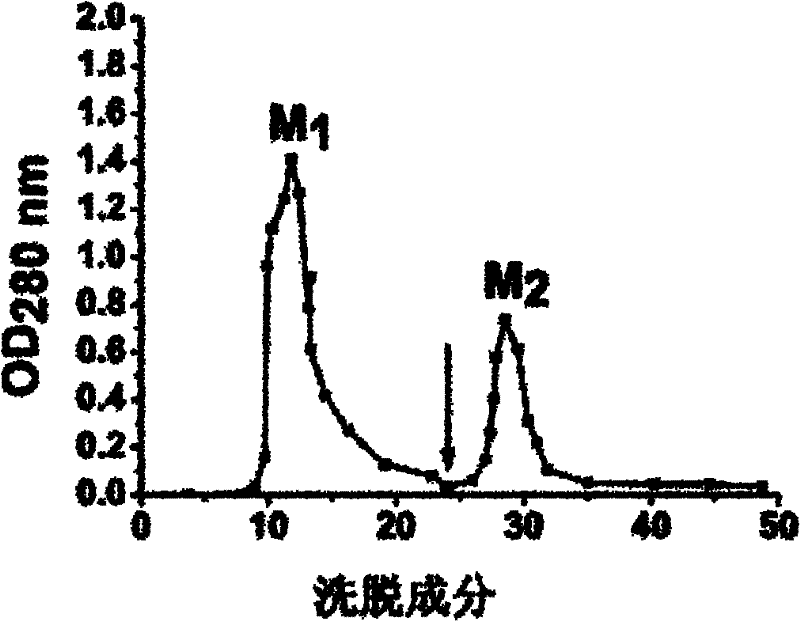
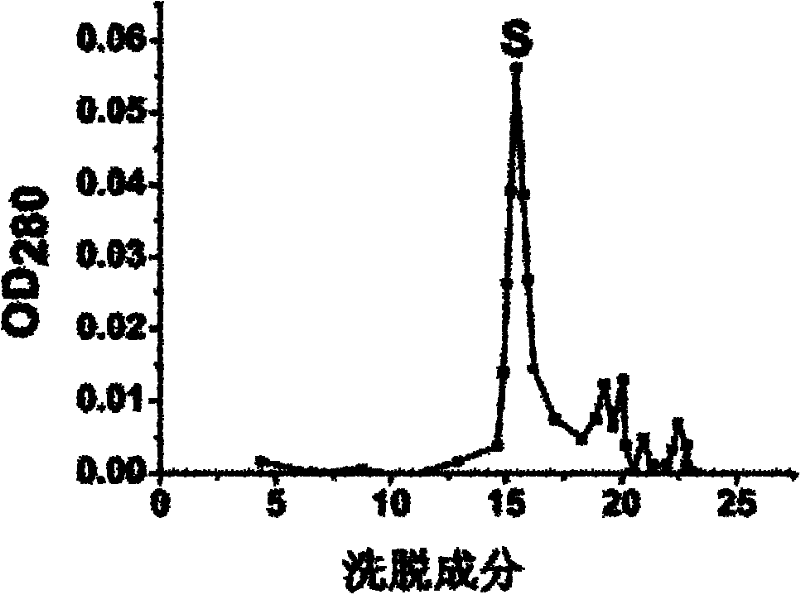
[0018] Wash the rhizomes and leaves of Fenugreek, homogenize it with a mixer until it becomes a paste, then add 2 times the volume of 0.2M sodium chloride solution of Fenugreek paste, and place it at 4°C for 12 hours, 4 Filter through a layer of gauze, centrifuge (4°C, 5,000g, 30min), collect the supernatant, and add solid ammonium sulfate to the obtained supernatant to reach 50% saturation, place it at 4°C for 6 hours, centrifuge (4°C, 10,000g , 20min) to remove the precipitate, continue to add ammonium sulfate to the obtained supernatant to 80% saturation, place it at 4°C for 12 hours, centrifuge (4°C, 10,000g, 20min) to collect the precipitate, dissolve the precipitate in distilled water, and dialyze to remove salt, and freeze-dried to obtain the crude lamb ear lectin protein....
Embodiment 2
[0026] Example 2: Gene cloning of the lectin of Elephantia spp. (using the method of rapid amplification of cDNA ends, ie 3' / 5'RACE method)
[0027] 1. Materials and methods
[0028] 1.1 Materials
[0029] A lectin protein sample of sageria: prepared by the method described in Example 1.
[0030] Plant material: The rhizomes and leaves of Fructus L. were collected from Pengzhou City, Sichuan Province, China, and were used directly or quickly frozen and stored at -70°C.
[0031] Bacterial strain: Escherichia coli Top10 (purchased from Invitrogen).
[0032] Plasmid vector: PUC18-T vector (purchased from TaKaRabao Biological Engineering Co., Ltd.).
[0033]Enzymes and kits: Taq DNA polymerase, MLV reverse transcriptase, TdT (nucleotide terminal transferase) RNase Inhibitor, RNase A (nucleotidase), RNA extraction kit, 3' / 5'RACE kit etc. were purchased from TaKaRa Bao Biological Engineering Co., Ltd. (Dalian, China), and the column gel recovery kit was purchased from Omiga Comp...
Embodiment 3
[0102] Example 3: Inhibitory effect of the lectin protein of Elephant spp. on agricultural harmful fungi
[0103] 1. Materials and Methods
[0104] 1.1 Materials
[0105] Pleurotus agglutinin protein: prepared by the method described in Example 1.
[0106] Harmful agricultural fungi: P. oryzae Cav., B. maydis, and F. oxysporum vasinfectum were purchased from Shanghai Fuzhong Biotechnology Development Co., Ltd.
[0107] 1.2 Method
[0108] paper method
[0109] 1.2.1 Preparation of filter paper
[0110] Choose filter paper with strong water absorption and uniform texture, use a puncher to punch into discs with a diameter of 6.25 mm, autoclave at 121 °C for 40 minutes, and dry them for later use.
[0111] 1.2.2 Preparation of bacterial suspension and potato agar culture plate
[0112] Add normal saline and glass beads after autoclaving to three sterile Erlenmeyer flasks respectively, and then take the activated rice blast fungus P.oryzae Cav. The slant strains of Fusarium...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com