Device and method for preparing gel microsphere and gel microsphere in which antitubercular agents can be injected
A technique for preparing gel microspheres and devices, which is applied in the direction of making drugs into special physical or ingestible devices, antibacterial drugs, and block delivery, etc., and can solve the complicated manufacturing process of anti-tuberculosis drug calcium alginate microspheres , Affect the production speed and quality of normal gel microspheres, hinder the droplet from contacting the gel solidification liquid, etc., achieve the effects of shortening the residence time, good atomization effect, and improving production speed and quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
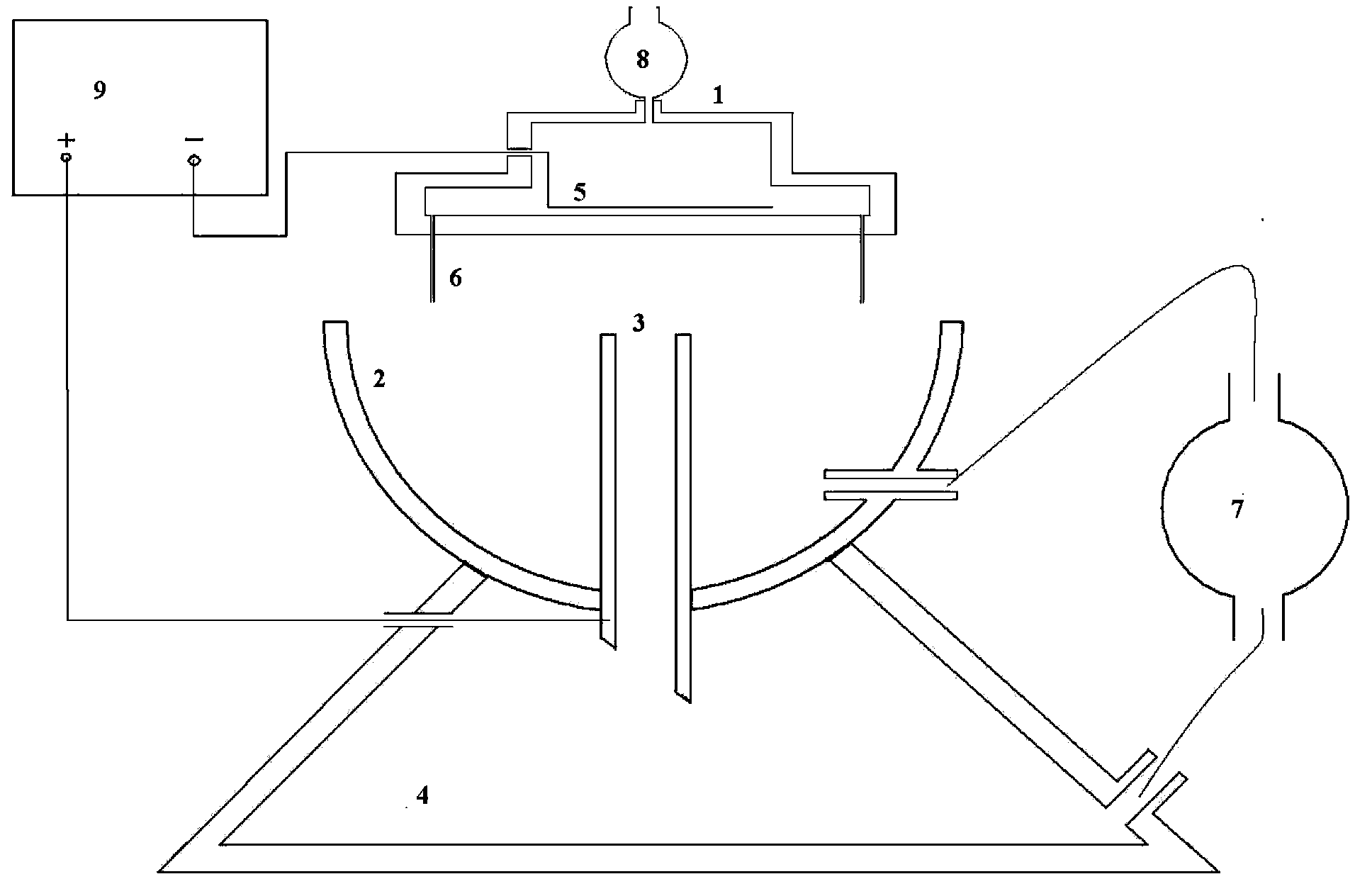
[0043] figure 1 Shown is a schematic structural diagram of a preferred embodiment of the gel microsphere preparation device of the present invention, the device includes: a porous nozzle 1, a collection cup 2, a collection pipe 3, a high-voltage electrostatic generator 9, a constant-flow pump 7, and a settling tank 4. Syringe pump 8 and infiltration electrode 5.
[0044] The upper part of the multi-hole spray head 1 has a liquid inlet, which is connected to the output end of the syringe pump 8, and the input end of the syringe pump 8 is connected to the drug-loaded sol storage device. The lower part of the porous nozzle has 12 nozzles 6 arranged in a ring. The nozzle 6 is a slender needle with a length of 6 cm to 20 cm. The inner diameter of the tip is 200 μm to 800 μm. The distance between the gel solidified liquid surface in the collection cup is 15mm to 60mm. There is a cavity in the porous nozzle 1, and a ring-shaped metal sheet is set in the cavity as the wetting electr...
Embodiment 2
[0063] Using the device and steps described in Example 1, changing the production formula and conditions, small particle size rifapentine gel microspheres can be obtained, and the specific process is as follows:
[0064] (1) Weigh 1.0g of rifapentine powder and 1.0g of stearic acid, dissolve in 8ml of ethyl acetate solvent at 50°C to obtain rifapentine stearic acid solution; weigh 1.8g of sodium alginate, dissolve In 90ml of distilled water, a 2.0wt% sodium alginate solution was obtained. The above rifapentine stearic acid solution was mixed with sodium alginate solution, and ultrasonically emulsified for 1 min to obtain rifapentine sol.
[0065] (2) Weigh 50 g of anhydrous calcium chloride, dissolve it in 2000 ml of distilled water, and prepare a 2.5 wt % calcium chloride solution to obtain a gel solidification solution.
[0066] (3) Put the rifapentine sol solution into the syringe pump. Set the voltage of the high-voltage electrostatic generator to 10000V, the inner diame...
Embodiment 3
[0072] Adopt the device described in embodiment 1 and similar steps, make rifabutin gel microsphere, concrete process is as follows:
[0073] (1) Weigh 2.5g of rifabutin powder and 2.5g of stearic acid, dissolve in 9.0ml ethyl acetate solvent at 50°C to obtain rifabutin stearic acid liquid; weigh 2.4g of sodium alginate, dissolve in 85ml of distilled water to obtain a 2.8wt% sodium alginate solution. The above rifabutin stearic acid solution and sodium alginate solution were heated to 60° C. and mixed to obtain rifabutin sol.
[0074] (2) Weigh 20 g of anhydrous calcium chloride, dissolve it in 2000 ml of distilled water, and prepare a 1 wt % calcium chloride solution to obtain a gel solidification solution.
[0075] (3) Fill the rifabutin sol into a syringe as a syringe pump. Set the voltage of the high-voltage electrostatic generator to 6000V, the inner diameter of the nozzle needle to 0.4mm, the distance between the tip of the nozzle needle and the surface of the gel soli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com