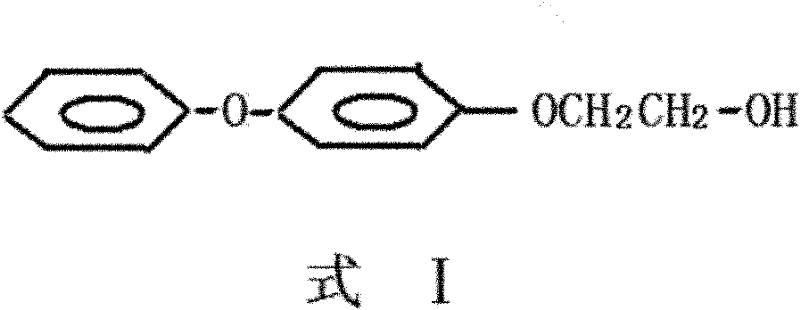
Preparation method for 2-(4-phenoxy phenoxy)ethanol
A technology of phenoxyphenoxy and p-phenoxyphenol, which is applied in the field of preparation of 2-ethanol, can solve the problems of complicated operation, low product yield, high material toxicity, etc., and achieve easy industrial implementation and high product yield High, stable quality results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
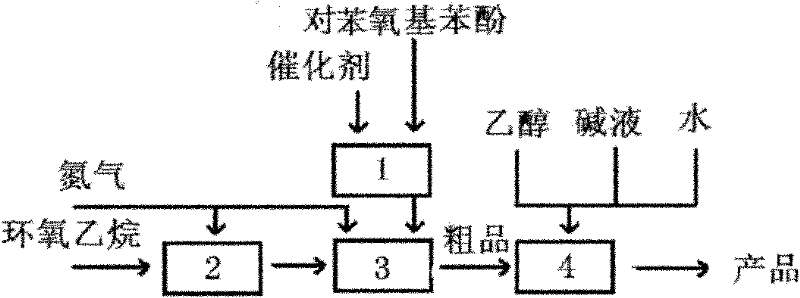
[0030] Such as figure 1 Shown, a kind of preparation method of 2-(4-phenoxyphenoxy)ethanol, the method comprises the steps:
[0031] (1) Ethoxylation reaction
[0032] Add 663g (3.56mol) of melted p-phenoxyphenol and 6g (0.107mol) of potassium hydroxide into a 2L stainless steel autoclave; then depressurize (-0.09mPa) and heat (80-90°C) for 40 minutes to remove Moisture contained in the material; decompress and replace the air in the reactor with nitrogen; gradually add 171g (3.89mol) ethylene oxide with nitrogen pressure to carry out ethoxylation reaction, control the reaction temperature to 85 ± 5 ° C, the reactor The pressure does not exceed 0.4mPa. After the addition of ethylene oxide was completed, the pressure in the kettle was reduced to 0, and then the heat preservation reaction was carried out for 30 minutes. Then the temperature in the kettle was lowered to 75-80° C., and the material was discharged to obtain 823 g of crude product. The purity of the crude produc...
Embodiment 2
[0036] A preparation method of 2-(4-phenoxyphenoxy)ethanol, the method may further comprise the steps:
[0037] (1) Add the melted p-phenoxyphenol (the purity of p-phenoxyphenol ≥ 99%) into the reactor;
[0038] (2) Vacuumize the material in the reactor to below -0.09mPa, heat at 80°C to 90°C for 30 minutes, and remove the contained water;
[0039] (3) Cool the contents of the reactor to 60° C. with cooling water, add triethylamine to the reactor, and the molar ratio of triethylamine to p-phenoxyphenol is 0.02:1;
[0040] (4) Negative pressure in the reaction kettle is made after vacuuming, and then filled with nitrogen to a pressure of 0, so replaced 3 times, and the pressure in the kettle is 0;
[0041] (5) Under a nitrogen atmosphere, ethylene oxide (ethylene oxide purity ≥ 99%) is gradually added to the reactor, the controlled reaction temperature is 85-105°C, and the pressure in the reactor is kept within the range of 0.3-0.5mPa. The molar ratio of oxyethane and p-pheno...
Embodiment 3
[0048] A preparation method of 2-(4-phenoxyphenoxy)ethanol, the method may further comprise the steps:
[0049] (1) Add the melted p-phenoxyphenol into the reactor, and add sodium methylate at the same time, the molar ratio of sodium methylate to p-phenoxyphenol is 0.04:1;
[0050] (2) Vacuumize the material in the reactor to below -0.09mPa, and heat it at 80°C to 90°C for 40 minutes to remove the contained water;
[0051] (3) Negative pressure in the reaction kettle is made after vacuuming, and then filled with nitrogen to a pressure of 0, so replaced 3 times, and the pressure in the kettle is 0;
[0052] (4) Under a nitrogen atmosphere, gradually add ethylene oxide to the reactor, control the reaction temperature to be 85-105°C, keep the pressure in the reactor in the range of 0.3-0.5mPa, the ratio of ethylene oxide and p-phenoxyphenol The molar ratio is 1.2:1;
[0053] (5) After the addition of ethylene oxide, maintain the reaction temperature, reduce the pressure in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com