Double-substituted 6-alkyl imidazolium-6-ammonium-beta-cyclodextrin with double positive electricity centers and preparation method thereof
A technology of cyclodextrin and alkylimidium, which is applied in the field of chiral resolution of racemates, can solve the problems of high cost and low yield, and achieve the effects of efficient resolution, good water solubility and excellent resolution ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
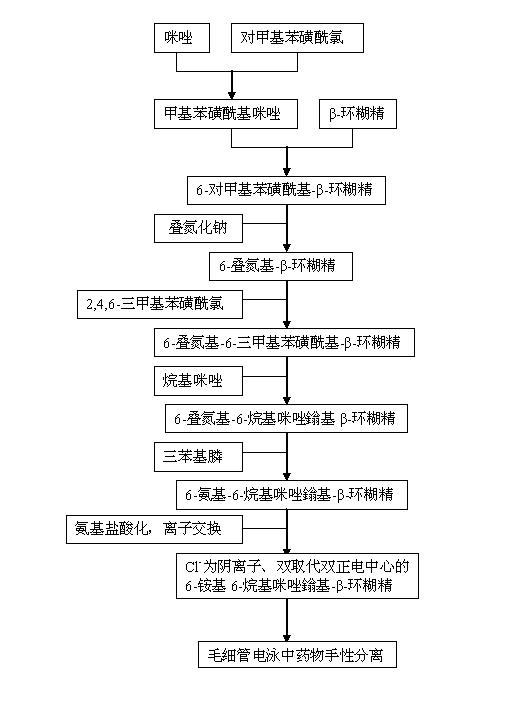
[0022] In conjunction with the accompanying drawings, the preparation method of the 6-alkylimidazolium-6-ammonium-β-cyclodextrin of the double-substituted double positive center of the present invention comprises the following steps:
[0023] In the first step, based on the mechanism of nucleophilic substitution, p-toluenesulfonyl chloride and imidazole often undergo a warm reaction in dichloromethane to obtain p-toluenesulfonyl imidazole;
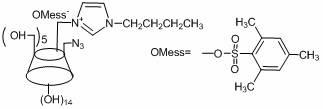
[0024] In the second step, the product p-toluenesulfonylimidazole obtained in the first step is placed in an aqueous solution in which β-cyclodextrin is dissolved, stirred and reacted at room temperature for 2 to 4 hours, and then 10 to 30% aqueous sodium hydroxide solution is added, and the resulting A small amount of precipitate; Utilize the low solubility of the generated Ts-CD at neutral or near neutral, add ammonium chloride to the filtrate to adjust its pH value to 6~8 to obtain a white solid substance, filter to obtain the product p-...
Embodiment 1
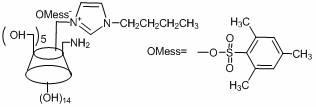
[0038] Implementation example 1: The preparation method of the present invention's double-substituted double positive center 6-ammonium-6-methylimidazolium-β-cyclodextrin chiral resolving agent comprises the following steps:
[0039] In the first step, take a 250 mL double-necked round-bottom flask and vacuumize it first, then blow it with nitrogen, weigh p-toluenesulfonyl chloride (6.57 g, 34.5 mmol) into the flask, then add 30 mL of dry dichloromethane, and stir to dissolve , then weigh imidazole (5.3 g, 77.8 mmol) and dissolve in 30 mL of dry dichloromethane solution, transfer to the dropping funnel, drop the above solution into the flask (1~2 drops / second), stir at room temperature overnight. After the reaction was completed and filtered, the filtrate was concentrated to ~10 mL and dropped into 40 mL of n-hexane solution, a white solid was precipitated, filtered under reduced pressure, the precipitate was washed with n-hexane, and vacuum-dried to obtain the product p-tolue...
Embodiment 2
[0050] Implementation Example 2: The preparation method of the present invention's double-substituted double-positive center 6-ammonium-6-ethylimidazolium-β-cyclodextrin chiral resolving agent comprises the following steps:
[0051] The first step is the same as the first step in Example 1;
[0052] The second step is the same as the second step of Example 1;
[0053] The third step is the same as the third step of Example 1;
[0054] The fourth step is the same as the fourth step of Example 1
[0055] The fifth step is to prepare ethylimidazole first: take a 50 mL two-necked flask, vacuum it first and then pass nitrogen, weigh imidazole (4 g, 58.8 mmol) into the flask, then measure 20 mL of dry ethanol into the flask, and stir for a while . Then sodium ethoxide (4.4 g, 64.6 mmol) was added into the flask, the temperature was raised to 40° C., and the mixture was stirred for half an hour. Measure bromoethane (7.04 g, 64.6 mmol) into the dropping funnel, drop it into the flas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com