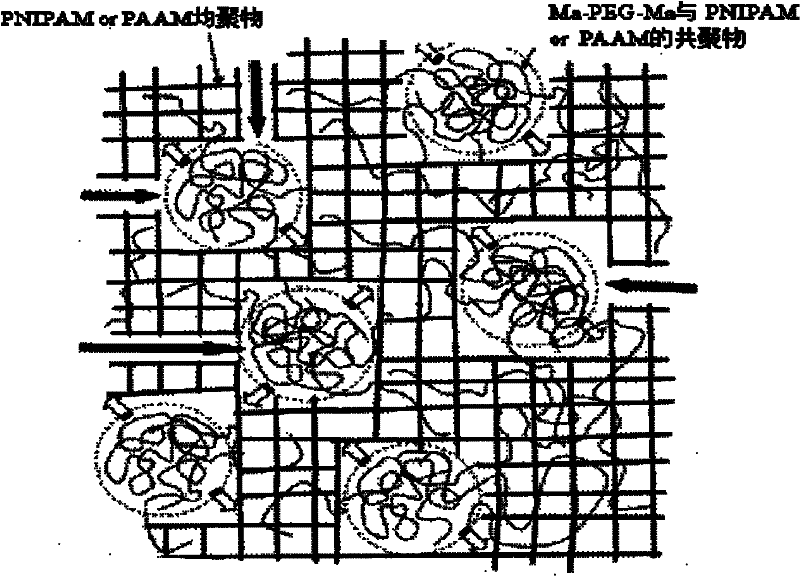
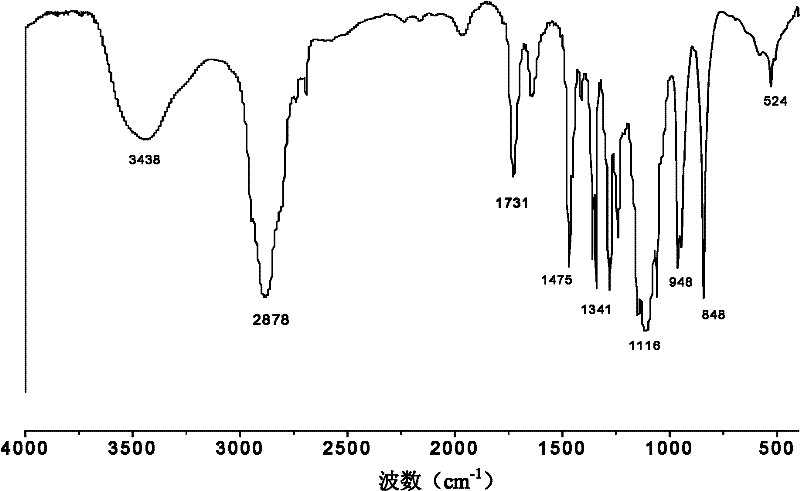
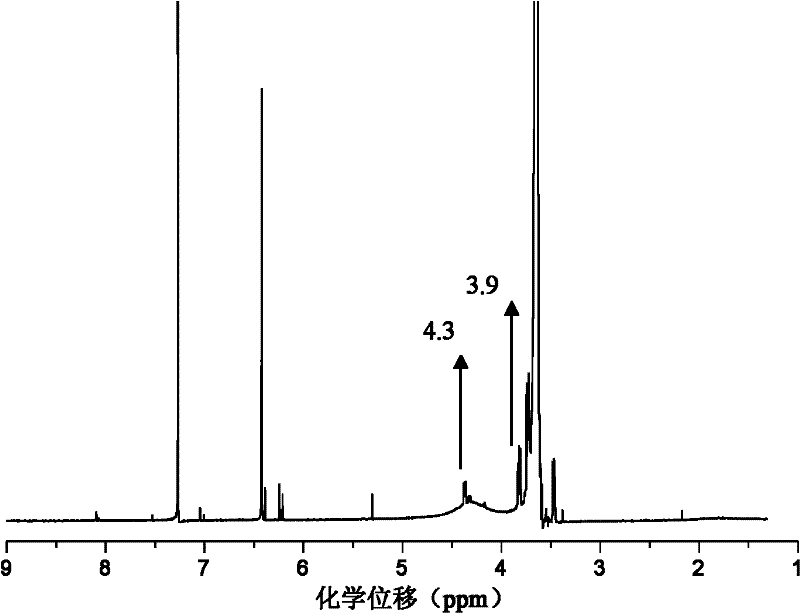
Double network polymer hydrogel and preparation method thereof
A polymer and hydrogel technology, applied in the field of biomedical materials, can solve the problems of unreported research, unsatisfactory response rate, low strength of electroresponsive gel, etc., and achieve the effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Step 1. Add 2g of polyethylene glycol (PEG) with a molecular weight of 200 after drying and removing water, 1.86g of maleic anhydride, and 5mL of toluene into the reaction vessel, seal it, vacuumize it, replace it with nitrogen, and stir at 65°C After 10 hours, the reaction was stopped, and the toluene in the reaction vessel was evaporated to dryness to obtain polyethylene glycol maleic anhydride diester.
[0047] Step 2. Dissolve polyethylene glycol maleic anhydride diester in 10-20 mL of dichloromethane, then precipitate in 100-200 mL of n-hexane, and obtain pure polyethylene glycol maleic anhydride diester after suction filtration;
[0048] Step 3. Mix 0.24g of product A with 0.083g of dried and purified N-isopropylacrylamide monomer, 0.067g of potassium persulfate, 0.0616g of N,N-methylenebisacrylamide (BIS), and add 3 ~4mL double distilled water, mix and stir evenly, heat up to 75°C in the reaction vessel and react for 2h to obtain DN polymer gel.
[0049] Step 4,...
Embodiment 2
[0054] Step 1. Add 4g of polyethylene glycol (PEG) with a molecular weight of 400 after drying and removing water, 2.41g of maleic anhydride, and 8mL of toluene into the reaction vessel, seal it, vacuumize it, replace it with nitrogen, and stir at 65°C After 10 hours, the reaction was stopped, and the solvent in the reaction vessel was evaporated to dryness to obtain polyethylene glycol maleic anhydride diester.
[0055] Step 2. Dissolve polyethylene glycol maleic anhydride diester in 12 mL of dichloromethane, then precipitate in 120 mL of anhydrous ether, and obtain product A after suction filtration;
[0056] Step 3. Mix 0.45g of product A with 0.148g of dry and purified acrylamide monomer, add 0.026g of 2,2-dimethoxy-2-phenylacetophenone (DMPA), and add 3.5mL of double distilled water , mixed and stirred evenly, moved to a mold, and irradiated under 500W ultraviolet light for 20 minutes to obtain a double-network polymer hydrogel.
[0057] Step 4, then cut the double netwo...
Embodiment 3
[0060] Step 1. Add 8g of polyethylene glycol (PEG) with a molecular weight of 800 after drying and removing water, 2.88g of maleic anhydride, and 14mL of toluene into the reaction vessel, seal it, vacuumize it, replace it with nitrogen, and stir at 70°C After 15 hours, the reaction was stopped, and the organic solvent in the reaction vessel was evaporated to dryness to obtain polyethylene glycol maleic anhydride diester.
[0061] Step 2. Dissolve polyethylene glycol maleic anhydride diester in 16 mL of dichloromethane, then precipitate in 160 mL of n-hexane, and obtain product A after suction filtration.
[0062] Step 3. Mix 0.79g of product A with 0.159g of dried and purified N-isopropylacrylamide monomer, 0.089g of ammonium persulfate, 0.0674g of ethylene glycol dimethacrylate (EGDMA), and add 3.8mL of dimethacrylate Subdistilled water, mixed and stirred evenly, heated up to 75°C in the reaction vessel and reacted for 2 hours to obtain DN polymer gel.
[0063] Step 4, then cu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum compressive strength | aaaaa | aaaaa |
| Maximum compressive strength | aaaaa | aaaaa |
| Maximum compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com