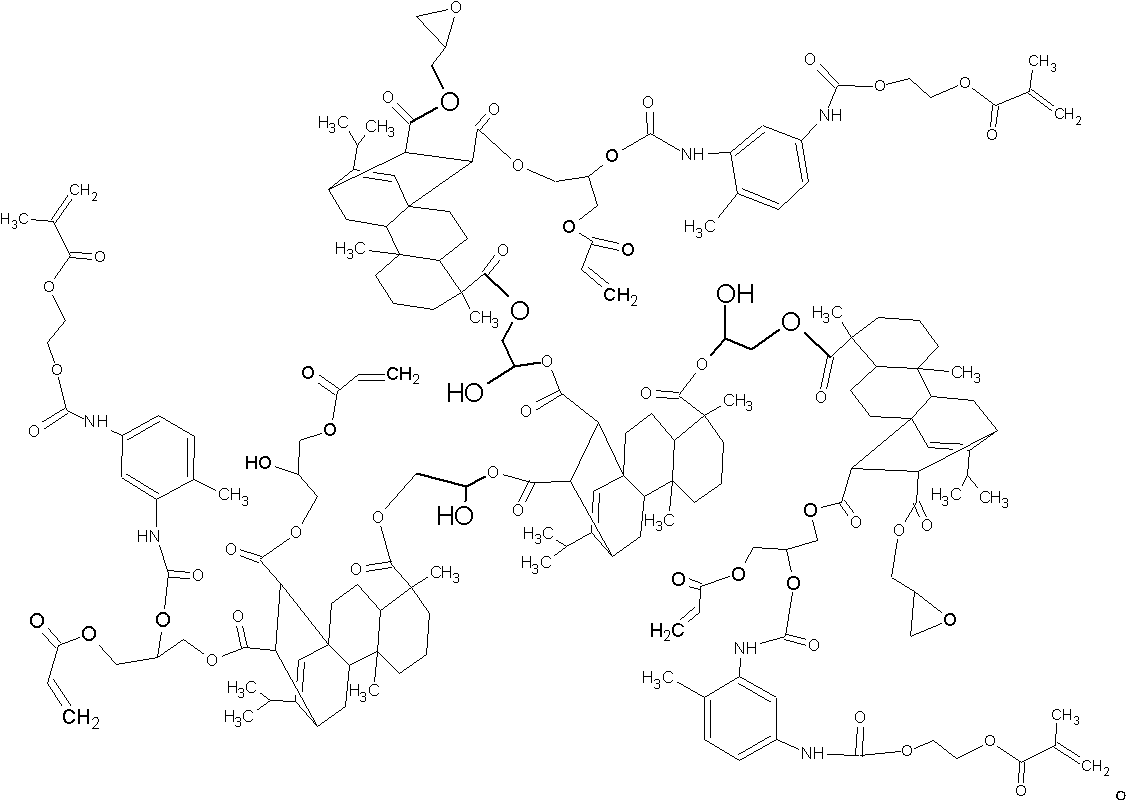
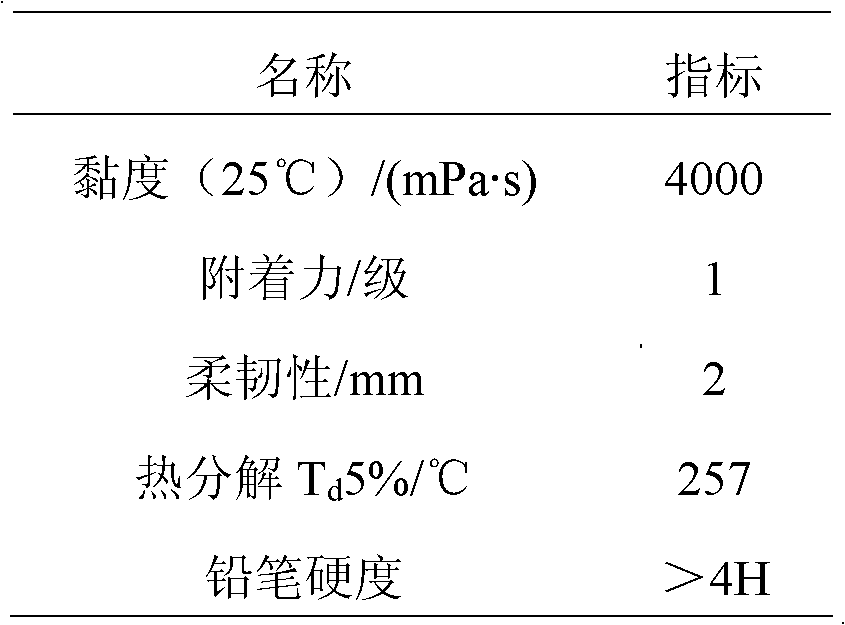
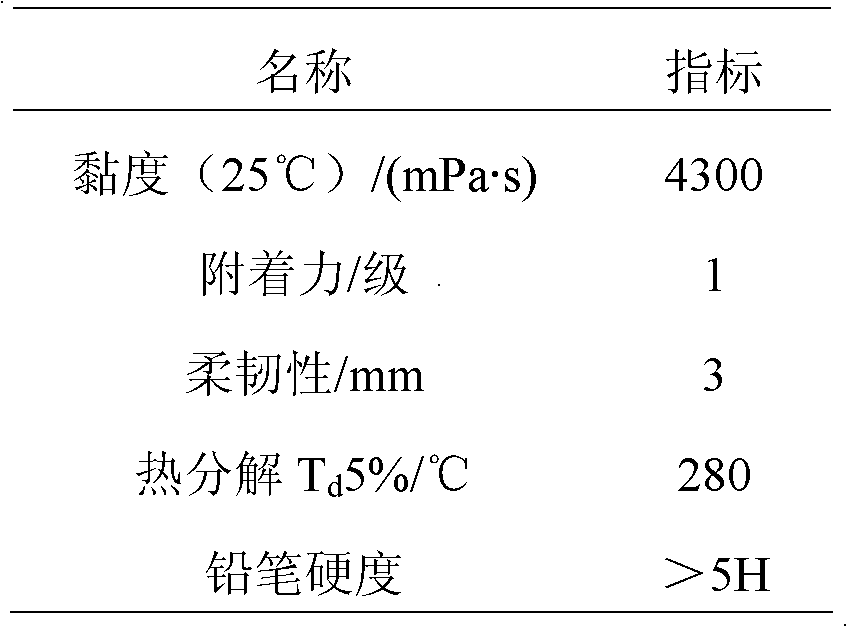
Method for preparing ultraviolet (UV)-curable polyisocyanate modified superbranched epoxy acrylate
A technology of epoxy acrylate and polyisocyanate, applied in organic chemistry, coating, etc., can solve the problems of high brittleness, poor light stability, and poor flexibility of epoxy resin cured film, so as to increase added value and increase flexibility , Improve the effect of water resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] a. Equipped with a spherical condenser, nitrogen enters the tube, add 200.7g maleopimaric acid into the four-necked flask with agitator, heat to 120°C, put in 100.0g maleopimaric acid triglycidyl ester, add 2.1g dibutyl Tin oxide is used as a catalyst to react until the acid value is 5mgKOH / g, and the hyperbranched compound HBR is obtained.
[0029] b. Cool down to 90°C, add 3.2g H 2 O, add 126.0 g of epichlorohydrin dropwise, and the dropping time is controlled at about 1 h, then add 3.3390 g of tetraethylammonium bromide (catalyst), and react until the acid value is 3 mgKOH / g to stop the reaction.
[0030] c. Then cool down the compound obtained in step b to 70°C, add 100.2g of NaOH aqueous solution with a concentration of 40%, add 6.3g of epichlorohydrin as a ring-closing solvent, keep the temperature at 20°C for 1h, filter the generated NaCl particles, and filter the filtrate through Wash with water until PH = 7, distill under reduced pressure to recover excess epi...
Embodiment 2
[0036] Except that the heating temperature in step a is 140° C., and the acid value is 8 mgKOH / g. In step b, the temperature is 110°C, the amount of catalyst potassium hydroxide is 4.7700g, and the acid value is 0.4mgKOH / g. In step c, the temperature was maintained at 35° C. for 1 h, and other conditions were the same as in Example 1.
Embodiment 3
[0038] Remove 229.0 g of maleopimaric acid in step a. In step b, add 5.0 g of water and 4.6210 g of dibutyltin oxide as a catalyst, and react until the acid value is 1 mgKOH / g. In step c, 91.8 g of NaOH aqueous solution with a concentration of 50% was added, and 10.0 g of epichlorohydrin was added. Acrylic acid 50.0g in step d, catalyst dibutyl tin oxide 3.5000g. In step e, TDI-HEMA is 135.0 g, and catalyst dibutyltin dilaurate is 0.9736 g. Other conditions are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com