Oral chronopharmacologic drug delivery mini-pill preparation of propranolol and its salts
A technology for propranolol hydrochloride and preparations is applied in the field of propranolol and its salt oral preparations, which can solve problems such as difficulty in taking medicine by patients, and achieve the effects of simple preparation process and improved compliance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
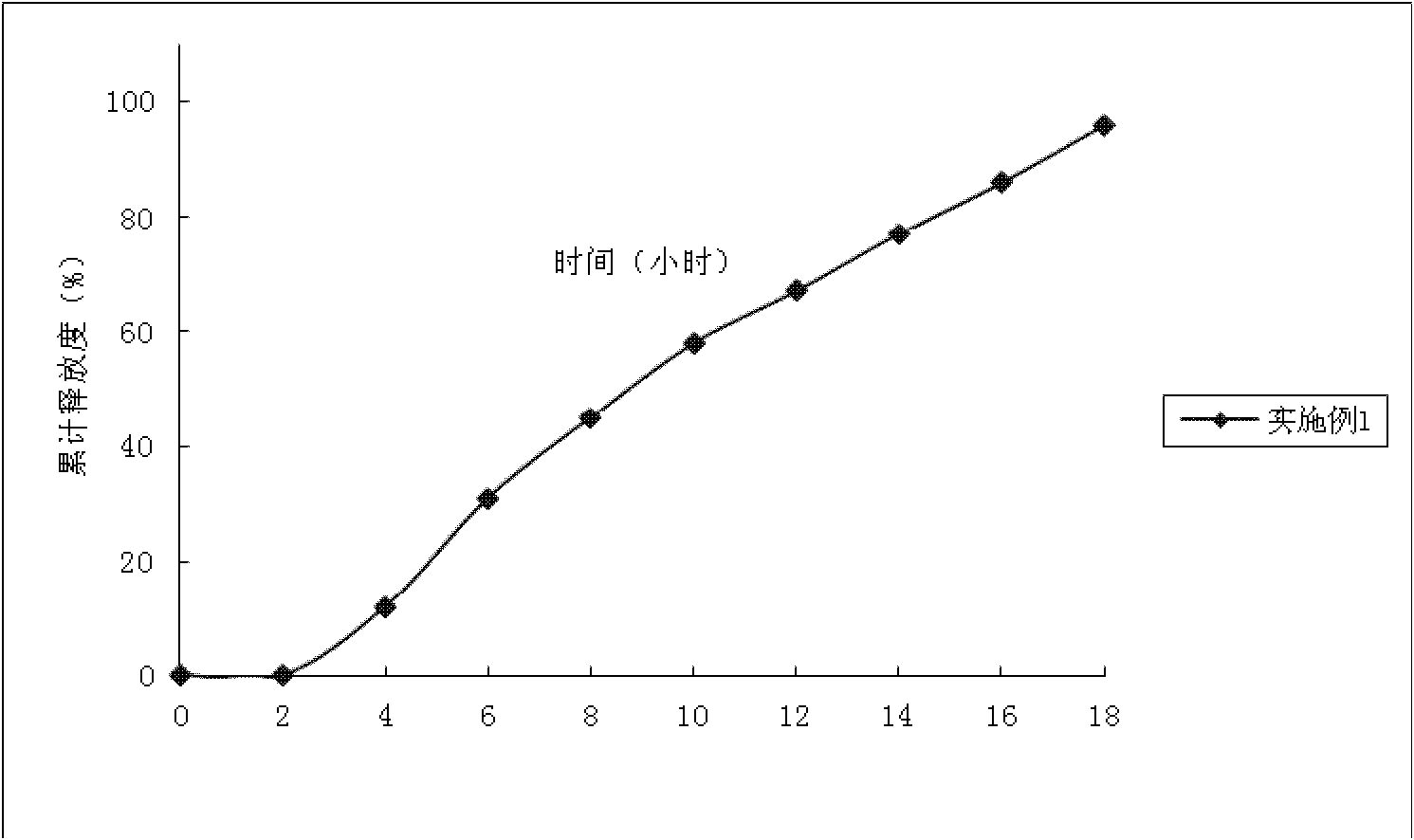
Embodiment 1
[0040] Example 1 The method for measuring the release of samples in different pH media is the same except that the replacement media is different from the previous one.
[0041] Example 1
[0042] Drug-containing immediate-release pill core prescription:
[0043] Starch sucrose blank core 200g
[0044] Propranolol Hydrochloride 240g
[0045] 80% ethanol 1kg
[0046] Preparation process: Dissolve isosorbide dinitrate in 95% ethanol, apply fluidized bed coating, temperature 32±1°C, flow rate 8mL / min, wrap propranolol hydrochloride on the blank core, and obtain Drug immediate release pill core. The average cumulative dissolution rate was 90% in 45 minutes.
[0047] Sustained-release layer prescription: Ethylcellulose 4% (w / v) 80% ethanol solution.
[0048] Preparation process: dissolve ethyl cellulose in 80% ethanol, apply fluidized bed coating, temperature 35±2°C, flow rate 8mL / min, wrap ethyl cellulose on drug-containing immediate-release pellet core, coating increases W...
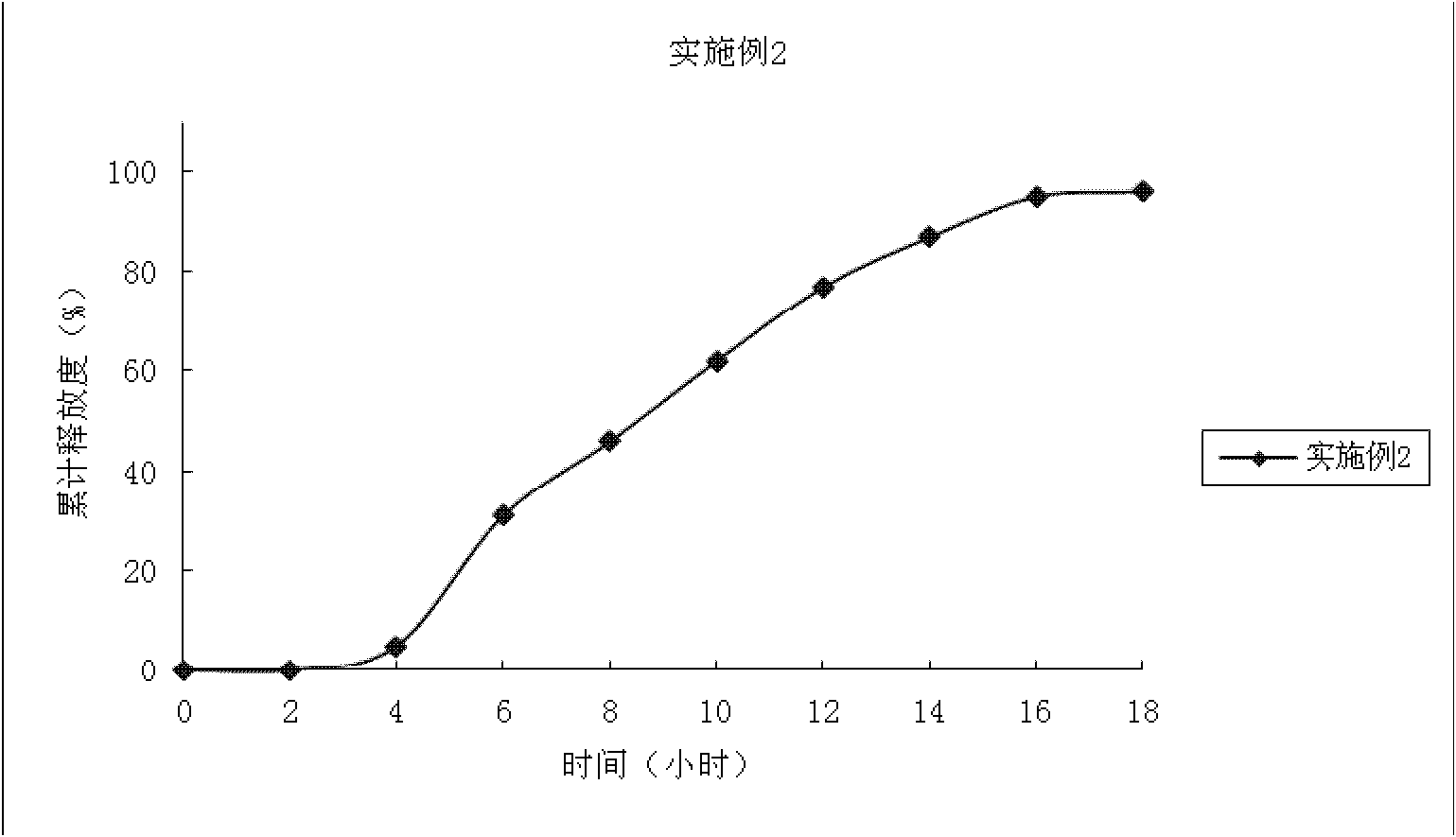
Embodiment 2
[0057] Drug-containing sustained-release pill core prescription:
[0058] Starch sucrose blank core 200g
[0059] Propranolol Hydrochloride 240g
[0060] 95% ethanol 1kg
[0061] The coating process is the same as in Example 1. The drug-containing immediate-release pellet core is obtained. The average cumulative dissolution rate was 90% in 45 minutes.
[0062] Sustained-release layer prescription: NE 30D is diluted with water to a solid content of 10%, and talcum powder accounting for 10% of the solid content is added as an anti-sticking agent, and stirred to make the mixture even.
[0063] Preparation process: Dilute a certain amount of NE 30D with water to a solid content of 10%, add talcum powder accounting for 10% of the solid content as an anti-adhesive agent, stir to mix evenly, and use a fluidized bed to coat at a temperature of 20±2°C. At a flow rate of 6 mL / min, NE 30D was coated on the drug-containing immediate-release pellet core, and the weight of the coating ...
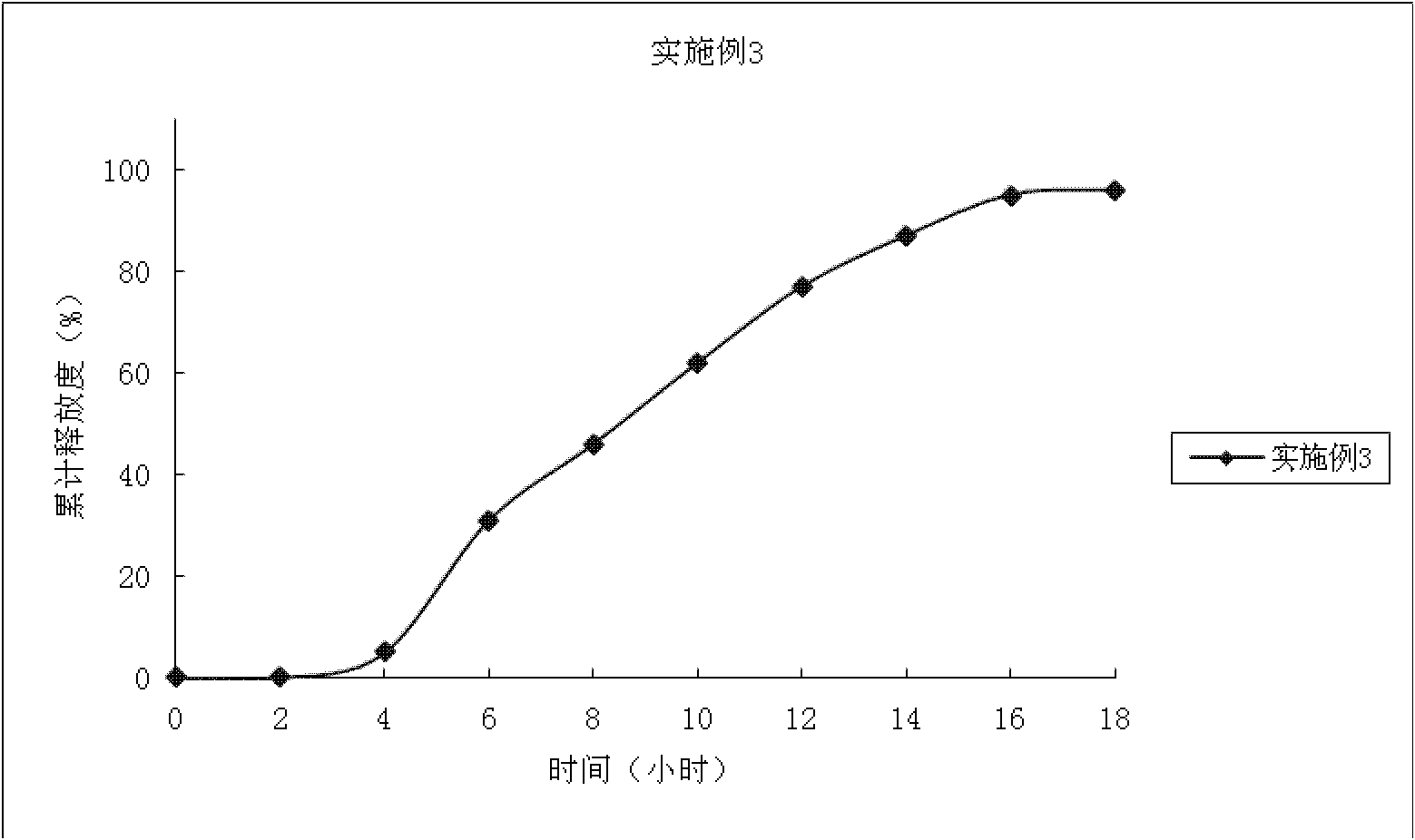
Embodiment 3
[0072] Whole medicated immediate-release pellet core formulation w / w(%)
[0073] Propranolol Hydrochloride 240g
[0074] Low-substituted hydroxypropyl cellulose 100g
[0075] Microcrystalline Cellulose 100g
[0076] Preparation process: add 5% HPMC in 70% ethanol solution to make soft material, extrude the soft material through the sieve plate of the extruder (aperture 0.8mm), put the strip-shaped particles in the spheronizer and spheronize, and dry the pellet core at 50°C After 5 hours, sieve the 18-24 mesh drug core to obtain the drug-containing immediate-release pellet core. The average cumulative dissolution rate was 5% in 45 minutes.
[0077] Sustained-release layer prescription: Ethylcellulose 4% (w / v) 80% ethanol solution.
[0078] Preparation process: dissolve ethyl cellulose in 80% ethanol, apply fluidized bed coating, temperature 35±2°C, flow rate 8mL / min, wrap ethyl cellulose on drug-containing immediate-release pellet core, coating increases Weight 8%, prepare...
PUM
| Property | Measurement | Unit |
|---|---|---|
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com