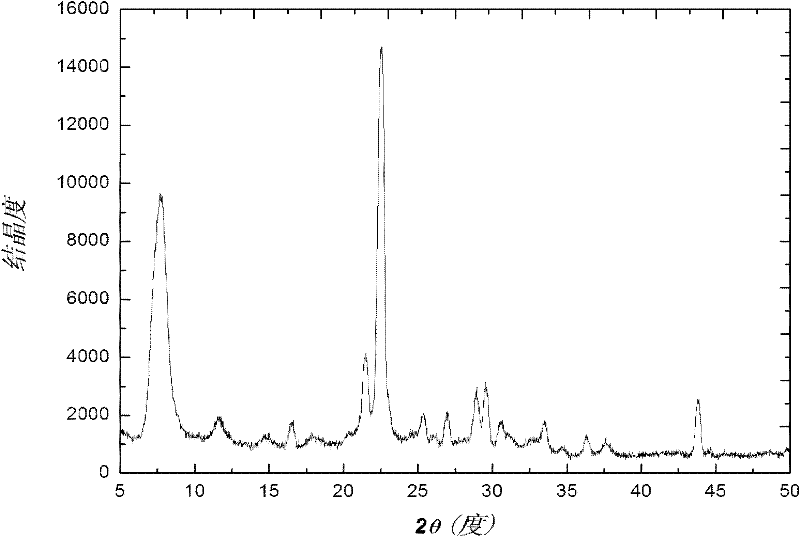
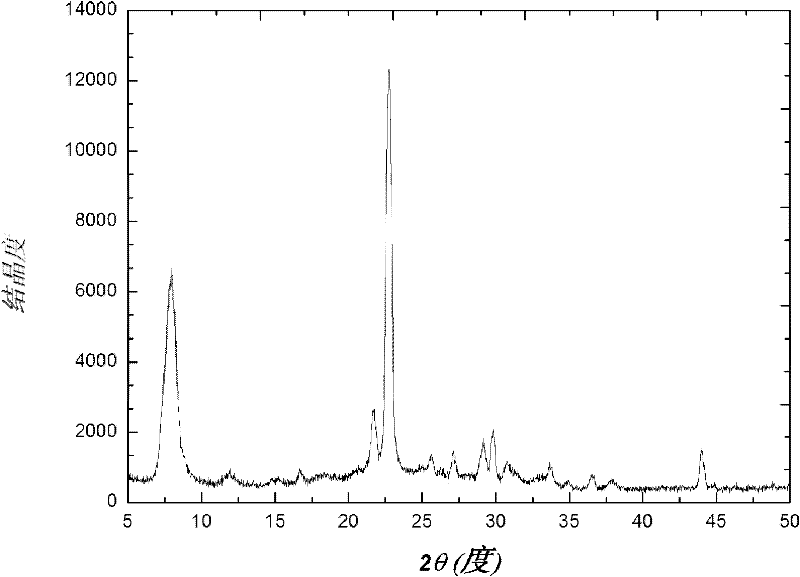
Method for synthesizing heteroatom Sn-beta zeolite
A heteroatom and beta zeolite technology, applied in chemical instruments and methods, crystalline aluminosilicate zeolite, molecular sieve catalyst, etc., can solve the problems of difficult stirring, high preparation cost, pollution, etc., and achieve easy recovery and recycling, and improve The effect of mechanical stirring performance and high-efficiency catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The initial molar ratio of heteroatom Sn-β zeolite synthesis is as follows: 1SiO 2 / 0.002SnO 2 / 0.6TEAOH / 0.6NH 4 F / 6.5H 2 O / 0.5EtOH, the specific synthesis process is as follows: 18g of white carbon black was added to 66.28g of tetraethylammonium hydroxide solution (TEAOH 40wt%), and stirred for about 2 hours until the white carbon black was completely dissolved. After dissolving 0.215g of tin tetrachloride pentahydrate in 0.5g of deionized water, it was added dropwise to the above-mentioned glue solution, and then 10ml of ethanol solution was added to the above-mentioned synthetic solution. After stirring for 1 hour, a solution formed by dissolving 6.66g of ammonium fluoride in 2.5g of deionized water and 5ml of ethanol was added, stirred vigorously, and a viscous gel was formed after about 30 minutes. The above gel was transferred into a small stainless steel synthesis kettle for static crystallization at 100°C. After 10 days, it was taken out and cooled, filtered...
Embodiment 2
[0028] The initial molar ratio of heteroatom Sn-β zeolite synthesis is as follows: 1SiO 2 / 0.002SnO 2 / 0.6TEAOH / 0.6NH 4 F / 6.5H 2 O / 0.5EtOH, the specific synthesis process is as follows: 18g of white carbon black was added to 66.28g of tetraethylammonium hydroxide solution (TEAOH 40wt%), and stirred for about 2 hours until the white carbon black was completely dissolved. After dissolving 0.215g of tin tetrachloride pentahydrate in 0.5g of deionized water, it was added dropwise to the above-mentioned glue solution, and then 10ml of ethanol solution was added to the above-mentioned synthetic solution. After stirring for 1 hour, a solution formed by dissolving 6.66g of ammonium fluoride in 2.5g of deionized water and 5ml of ethanol was added, stirred vigorously, and a viscous gel was formed after about 30 minutes. The above gel was transferred into a small stainless steel synthesis kettle for static crystallization at 180°C. After 10 days, it was taken out and cooled, filtered...
Embodiment 3
[0030] The initial molar ratio of heteroatom Sn-β zeolite synthesis is as follows: 1SiO 2 / 0.002SnO 2 / 0.6TEAOH / 0.6NH 4 F / 6.5H 2 O / 0.5EtOH, the specific synthesis process is as follows: 18g of white carbon black was added to 66.28g of tetraethylammonium hydroxide solution (TEAOH 40wt%), and stirred for about 2 hours until the white carbon black was completely dissolved. After dissolving 0.215g of tin tetrachloride pentahydrate in 0.5g of deionized water, it was added dropwise to the above-mentioned glue solution, and then 10ml of ethanol solution was added to the above-mentioned synthetic solution. After stirring for 1 hour, a solution formed by dissolving 6.66g of ammonium fluoride in 2.5g of deionized water and 5ml of ethanol was added, stirred vigorously, and a viscous gel was formed after about 30 minutes. The above gel was transferred into a small stainless steel synthesis kettle for static crystallization at 140°C. After 10 days, it was taken out and cooled, filtered...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com