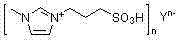A kind of method that adopts oxidative halogenation method to prepare haloaryl compound
A technology of halogenated aryl and compound, which is applied in the field of fine chemical synthesis to achieve the effects of good selectivity, mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6
[0012] Add 2 mmol catalyst, 5 mmol anisole, 10 mmol sodium bromide, 10 mmol hydrogen peroxide (30% aqueous solution) and 3 mL water into the round bottom flask, react for 3 hours at room temperature and constant speed, and then stand still , Extract twice with 15mL ether, combine the ether extracts, analyze with gas chromatography to determine the yield of bromoanisole; after gas chromatography analysis, the ether phase is saturated with NaHCO 3 The solution and water are washed separately, dried with anhydrous sodium sulfate, distilled to remove the ether, and then distilled under reduced pressure to obtain pure p-bromoanisole. The lower water phase (containing catalyst) can continue to be recycled. Table 1 shows the results of the aqueous oxidative bromination reaction of anisole and sodium bromide using different bifunctional imidazolium ionic liquids as catalysts.
[0013] Table 1 Aqueous oxidation bromination results of anisole and sodium bromide with different bifunctional...
Embodiment 7~15
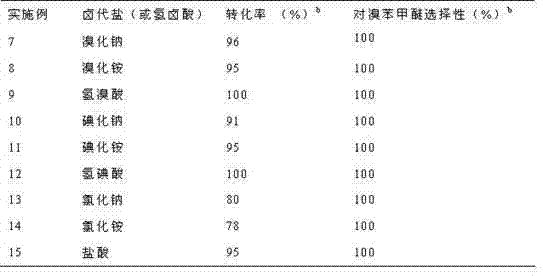
[0020] Table 2 gives the catalyst 6 Under the action of, the oxidative halogenation reaction of different halogenated salts or halogen acids and anisole in the water phase results. Add 2 mmol catalyst 6 , 5 mmol anisole, 10 mmol halogenated salt (or halogen acid), 10 mmol hydrogen peroxide (30% aqueous solution) and 3 mL water were added to the round bottom flask, and the oxidation was carried out according to the steps and conditions of Examples 1 to 6. Halogenation reaction.
[0021] Table 2 catalyst 6 Aqueous oxidation and halogenation reaction results of different halogenated salts or halogen acids and anisole under action a
[0022]
[0023] a 2 mmol catalyst 6 , 5 mmol anisole, 10 mmol halogenated salt (or hydrohalic acid), 10 mmol hydrogen peroxide (30%), 3 mL water, room temperature, reaction time 3 hours.
[0024] b Gas chromatography analysis.
[0025]
Embodiment 16~30
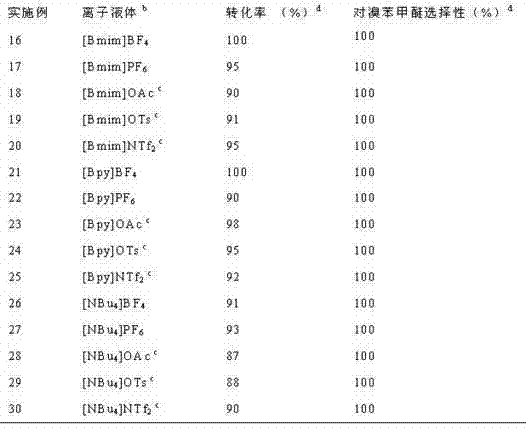
[0027] Table 3 gives the catalyst 6 The oxidative bromination reaction results of hydrobromic acid and anisole in different ionic liquids under the action of. Add 0.5 mmol catalyst 6 , 5 mmol anisole, 5 mmol hydrobromic acid, 7.5 mmol hydrogen peroxide (30% aqueous solution) and 3 mL different ionic liquids were added to the round-bottom flask, and the oxidative bromination reaction was carried out according to the steps and conditions of Examples 1 to 6. .
[0028] table 3 catalyst 6 Oxidative bromination results of hydrobromic acid and anisole in different ionic liquids under action a
[0029]
[0030] a 0.5 mmol catalyst 6 , 5 mmol anisole, 5 mmol hydrobromic acid, 7.5 mmol hydrogen peroxide (30%), 3 mL ionic liquid, room temperature, reaction time 3 hours.
[0031] b Bmim=1-methyl-3-butylimidazole quaternary ammonium cation; Bpy=N-butylpyridine quaternary ammonium cation.
[0032] c OAc=Acetate, OTs=Toluenesulfonate, NTf 2 = Trifluoromethanesulfonimide anion.
[0033] d Gas c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com