Method for preparing carbon coated nanometer LiFePO4
A lithium iron phosphate and nano iron phosphate technology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems affecting the large-scale production and application of lithium iron phosphate materials, poor conductivity of lithium iron phosphate cathode materials, Cathode material purity and non-uniform particle size, etc., to achieve the effect of easy implementation, improved ion mobility and electrical conductivity, and wide particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1) Take nano-iron phosphate, nano-lithium carbonate and stearic acid according to the ratio of molar ratio Fe:Li:C=1:1:2.5, wherein iron phosphate is 0.005mol. Dissolving stearic acid in ethanol, and then adding nano-iron phosphate and nano-lithium carbonate thereto. The resulting suspension was sonicated for 15 min to obtain a stable and uniform nanofluid. The nanofluid was dried in a constant temperature oven at 80° C. for 30 minutes, and the solvent was removed to obtain a solid particle reaction mixture.
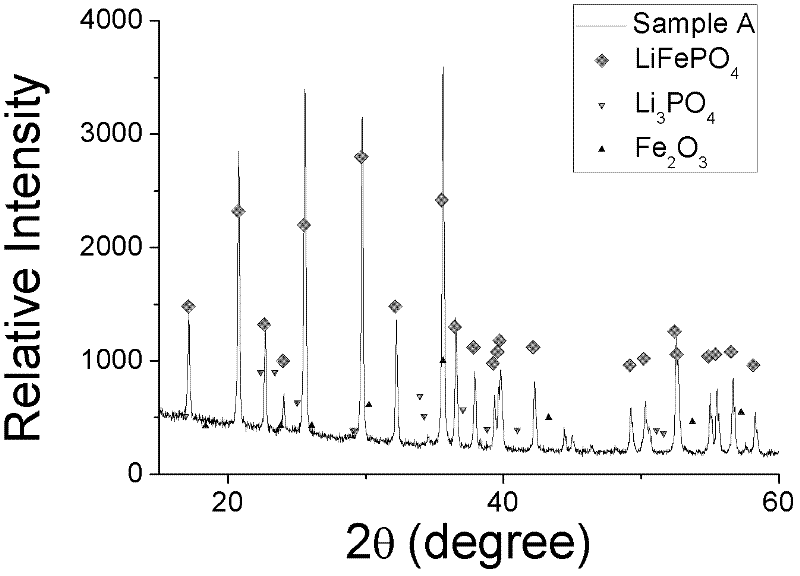
[0028] 2) The obtained solid particle reaction mixture was placed in a tubular reactor, pre-calcined at 300° C. for 1 h in an inert gas Ar atmosphere atmosphere, and calcined at 700° C. for 6 h, wherein the heating rate was 5 K / min. Lithium iron phosphate particles were obtained after natural cooling, which was designated as sample A.
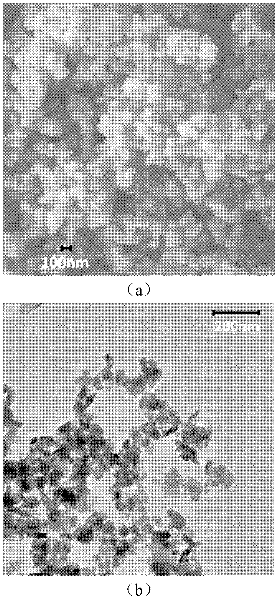
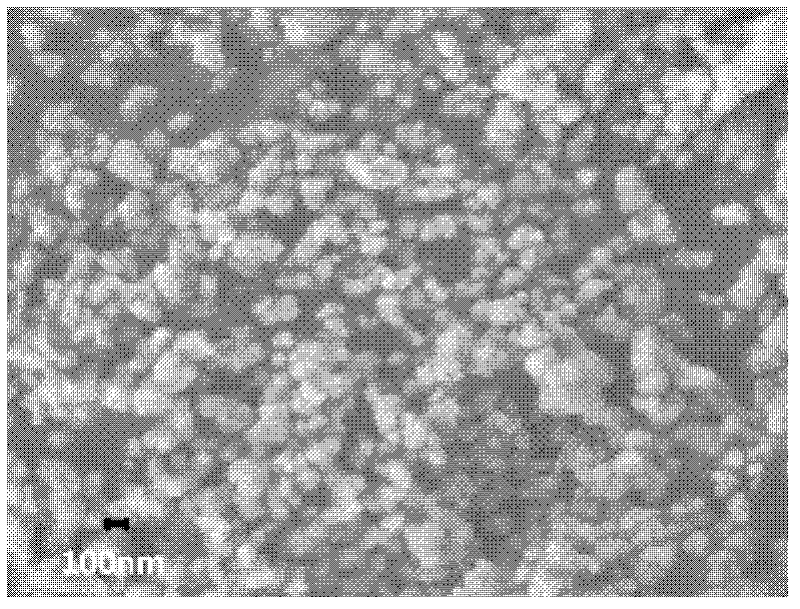
[0029] figure 1 The SEM image of the nano-iron phosphate and the TEM image of the nano-lithium carbonate used in Example 1 sho...
Embodiment 2
[0031] 1) Take nano-iron phosphate, nano-lithium carbonate and vitamin C according to the ratio of molar ratio Fe:Li:C=1:1:5, wherein iron phosphate is 0.005mol. The vitamin C is dissolved in an aqueous solution of acetone containing 50% of acetone, and then nano iron phosphate and nano lithium carbonate are added thereto. The resulting suspension was sonicated for 15 min to obtain a stable and uniform nanofluid. Send the nanofluid into a vacuum desiccator to remove the solvent to obtain a solid particle reaction mixture.
[0032] 2) The obtained solid particle reaction mixture was placed in a tubular reactor, pre-calcined at 300° C. for 1 h in an inert gas Ar atmosphere atmosphere, and calcined at 700° C. for 6 h, wherein the heating rate was 5 K / min. Lithium iron phosphate particles were obtained after natural cooling.
Embodiment 3
[0034] 1) Take nano-iron phosphate, nano-lithium carbonate and oxalic acid according to the ratio of molar ratio Fe:Li:C=1:1:3, wherein iron phosphate is 0.005mol. Dissolving oxalic acid in isopropanol aqueous solution containing 30% isopropanol, and then adding nano iron phosphate and nano lithium carbonate thereto. The resulting suspension was mechanically ground for 15 min to obtain a stable and uniform nanofluid. The nanofluid is sent to a flash evaporator to remove the solvent to obtain a solid particle reaction mixture.
[0035] 2) The obtained solid particle reaction mixture is placed in a tubular reactor, pre-calcined at 300° C. for 1 h in a hydrogen atmosphere, and calcined at 700° C. for 6 h, wherein the heating rate is 10 K / min. Lithium iron phosphate particles were obtained after natural cooling.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com