Secnidazole vaginal suppository and its preparation process
A technique for secnidazole and vaginal suppositories, which is applied in the field of preparation technology of secnidazole vaginal suppositories, can solve the problems of complex components and preparation processes, liver function damage, complex preparation processes, etc., and achieve simple proportioning and curative effect Good, simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
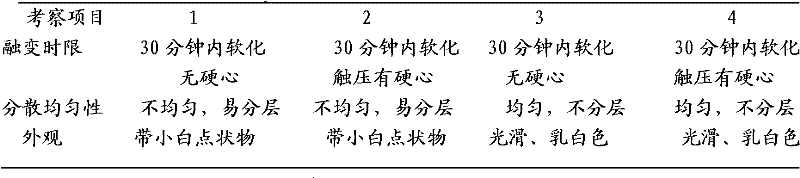
[0026] The implementation of the present invention will be described in detail below through the examples, but they are not construed as limiting the present invention, but only as examples, and will become clearer and easier to understand by illustrating the advantages of the present invention.
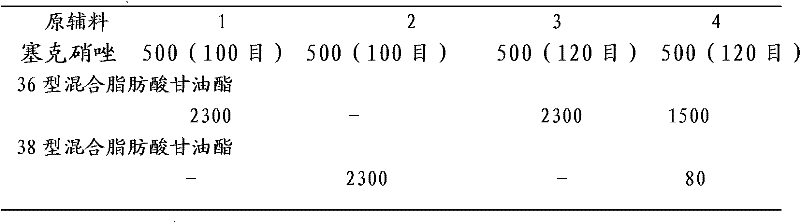
[0027] Secnidazole vaginal suppository of the present invention, it is made up of following components: major ingredient secnidazole 500g, auxiliary material 36 type mixed fatty acid glycerides 2300g, make 1000 suppositories, each grain weighs 2.8 grams,
[0028] Mixed fatty acid glycerides are mixtures of triglycerides, diglycerides, and monoglycerides. According to the different melting points, it can be divided into four types: type 34, type 36, type 38 and type 40. Among them, the melting point of type 34 is 33-35°C, the melting point of type 36 is 35-37°C, the melting point of type 38 is 37-39°C, and the melting point of type 40 is 39-41°C.
[0029] The molecular formulas of tr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com