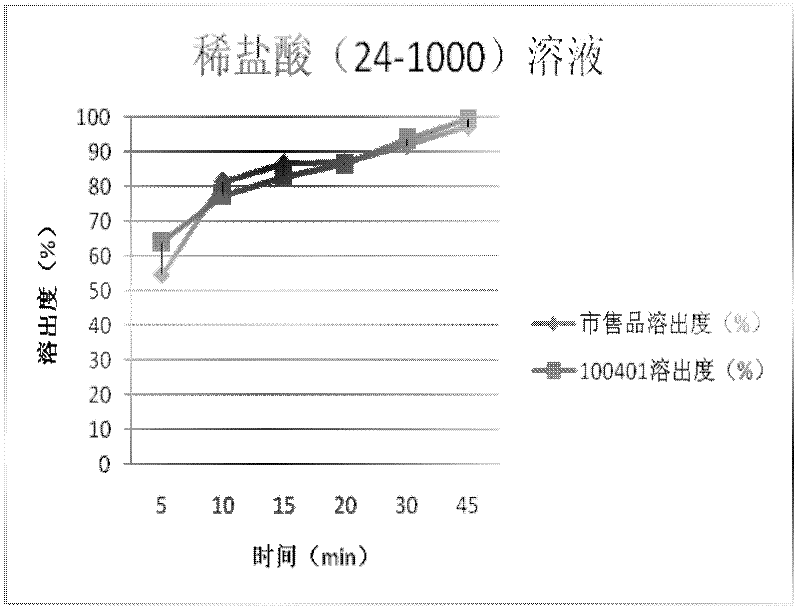
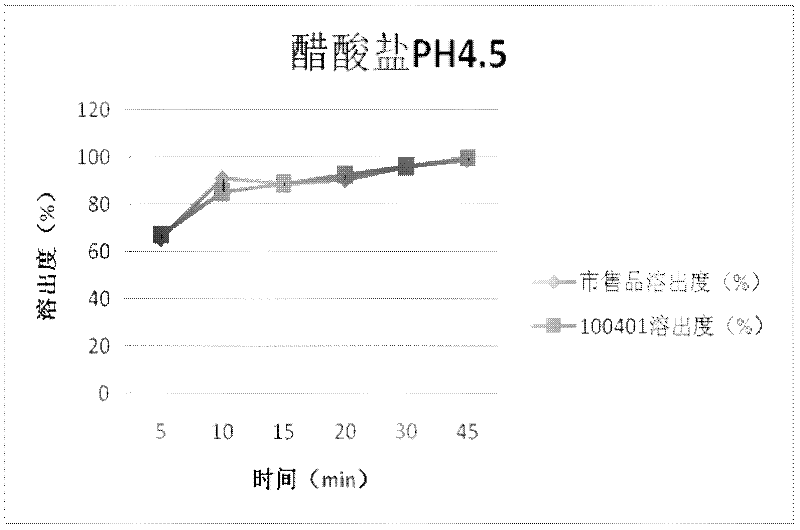
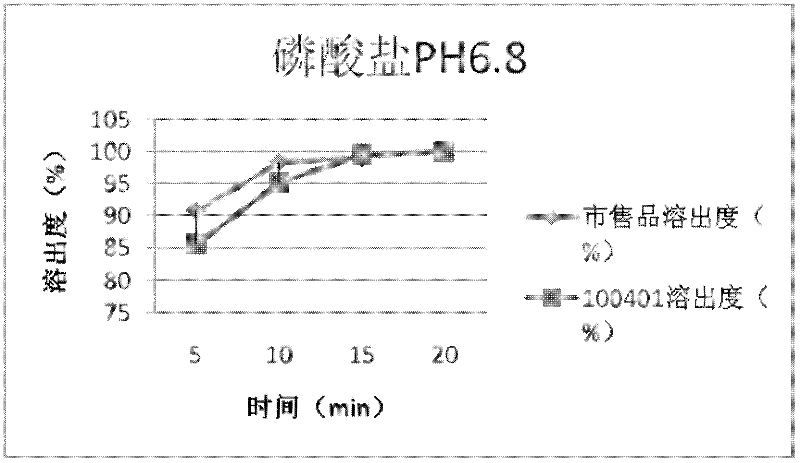
Cefdinir capsule and preparation method thereof
A technology of dini capsule and cefdinir, which is applied in the field of cefdinir capsule and its preparation, can solve the ineffectiveness of Pseudomonas aeruginosa, Pseudomonas cepacia, Acinetobacter, enterococcus and Listeria monocytogenes, It is also ineffective against Legionella and anaerobic bacteria, including Bacteroides and Clostridium, and achieves the effects of good water solubility, simple preparation process and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
[0044] (1) pass the raw and auxiliary materials through a 100-mesh sieve for subsequent use;
[0045] (2) Weigh according to the prescription amount, and mix cefdinir and auxiliary materials evenly;
[0046] (3) Intermediate detection;
[0047] (4) Capsule filling, polishing, and packaging to obtain cefdinir capsules with a specification of 100 mg (calculated as cefdinir).
Embodiment 2
[0049]
[0050](1) The raw material, croscarmellose sodium, and poloxamer are respectively passed through a 100-mesh sieve for subsequent use;
[0051] (2) Weigh the raw and auxiliary materials according to the prescription amount, mix cefdinir with croscarmellose sodium and poloxamer evenly, add 40% ethanol in an appropriate amount to make a soft material, granulate with a 40-mesh sieve, 45-55 Dry between, 40 mesh sieves granulate, add the magnesium stearate of recipe quantity;
[0052] (3) Intermediate detection;
[0053] (4) Capsule filling, polishing, and packaging to obtain cefdinir capsules with a specification of 100 mg (calculated as cefdinir).
Embodiment 3
[0055]
[0056] (1) pass the raw and auxiliary materials through a 100-mesh sieve for subsequent use;
[0057] (2) Weigh according to the prescription amount, and mix cefdinir and auxiliary materials evenly;
[0058] (3) Intermediate detection;
[0059] (4) Capsule filling, polishing, and packaging to obtain cefdinir capsules with a specification of 50 mg (calculated as cefdinir).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com