The method that utilizes magnesium sulfate raw material to prepare magnesium hydroxide
A technology of magnesium hydroxide and magnesium sulfate, applied in directions such as magnesium hydroxide, can solve the problems of expensive organic amines, and achieve the effects of low price, convenient operation and small circulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
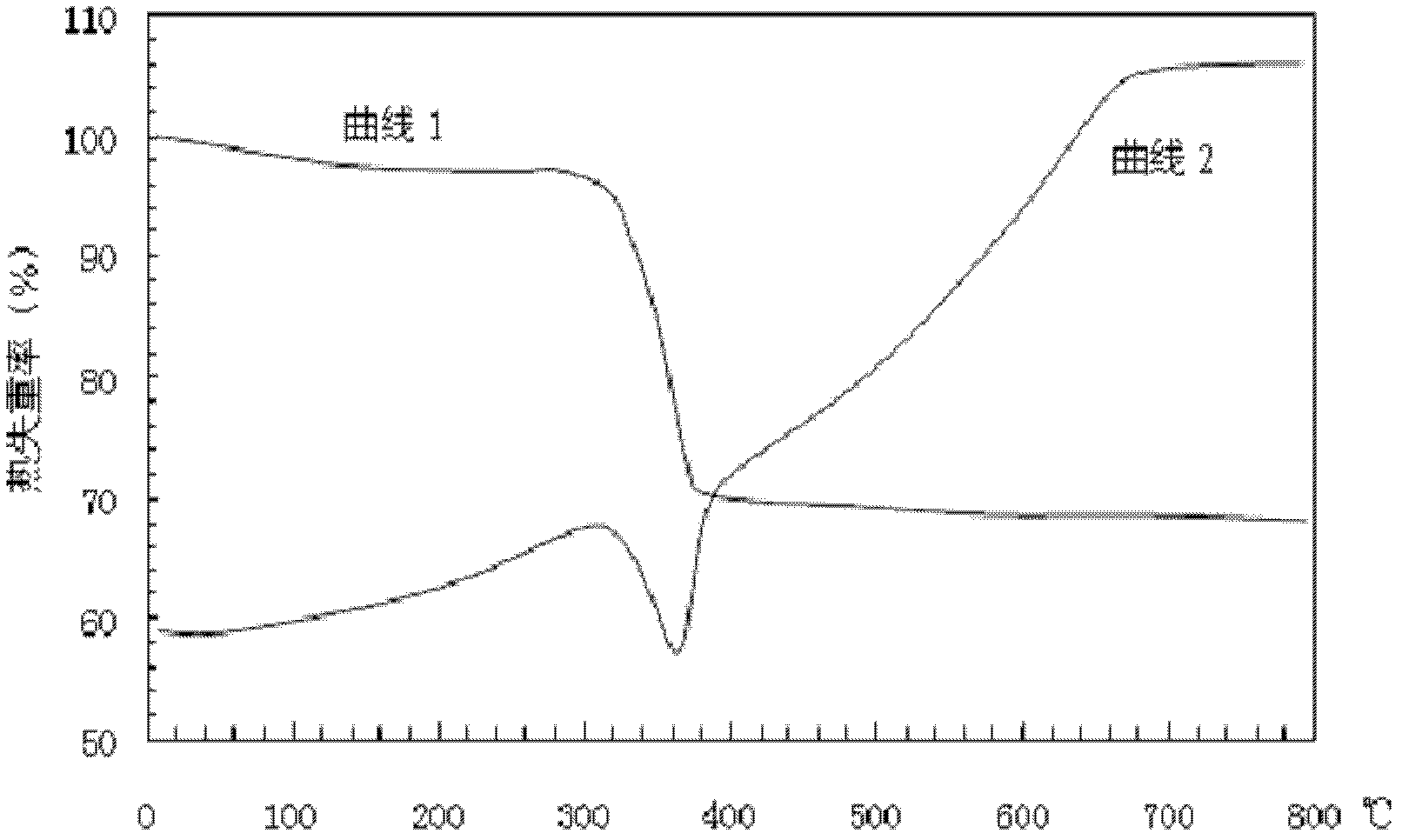
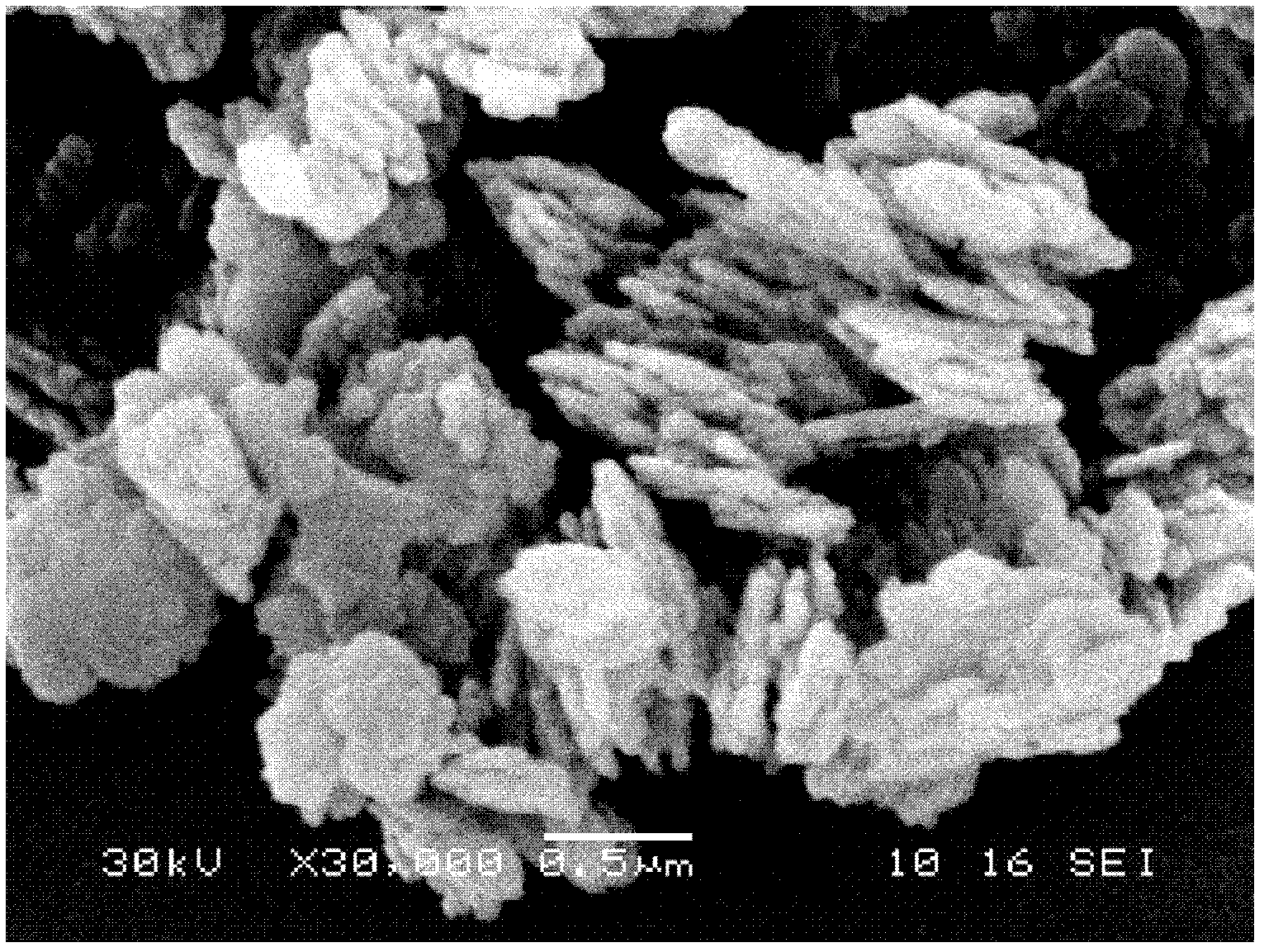
Image
Examples
Embodiment 1
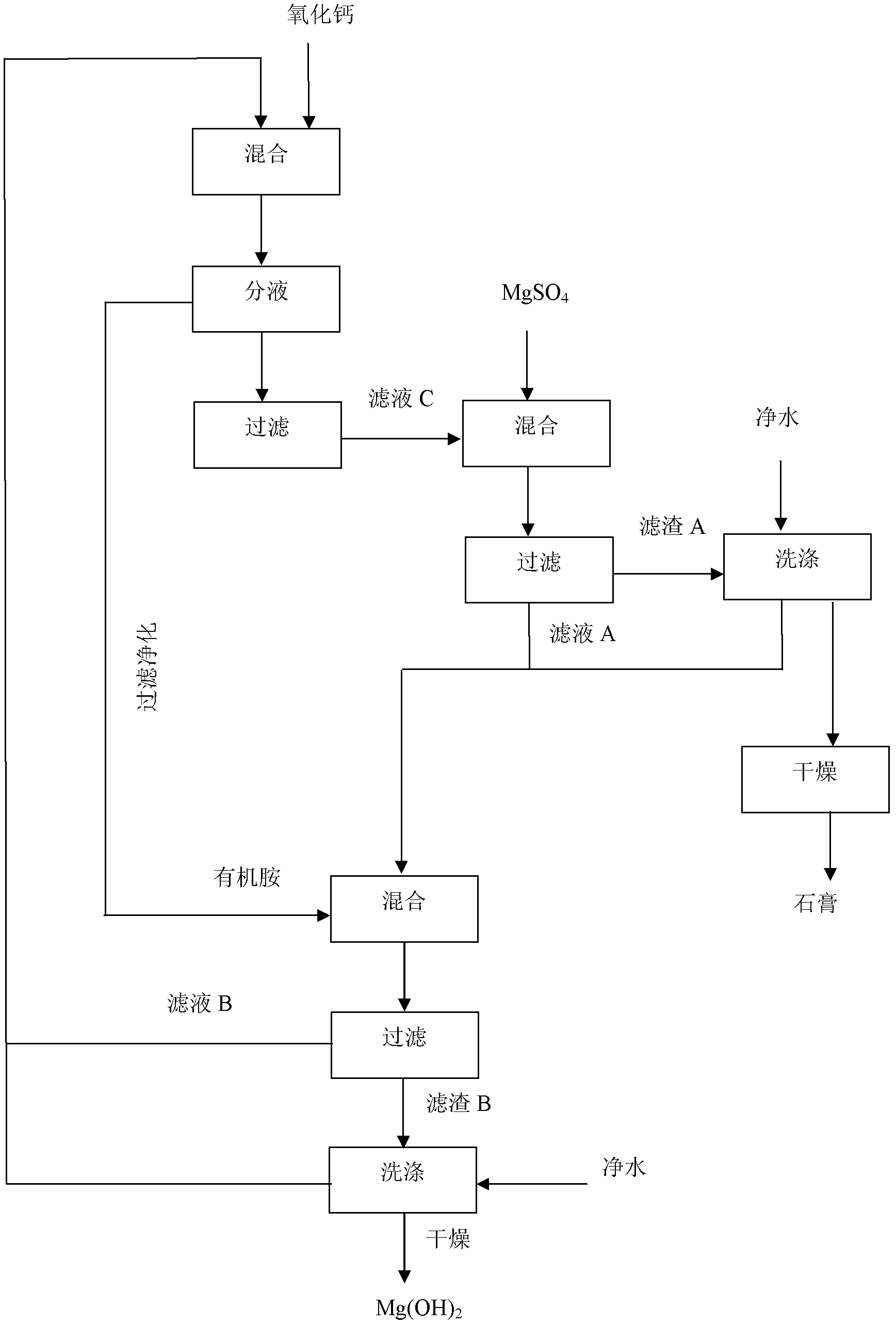
[0028] The raw materials used in the present invention include a. 1 part of calcium nitrate, which is 1 mol; b. 1 part of organic amine, which is di-n-butylamine, which is 2 mol; Calcium content is 1mol; d. 2 parts of magnesium sulfate raw material, the magnesium sulfate content is measured before use, each part is 1mol.
[0029] The start-up step of the process cycle of the present invention is firstly preparing calcium nitrate into a 1mol / L solution, then slowly adding the magnesium sulfate raw material into the solution, and then filtering to obtain filter residue A (i.e. raw gypsum) and filtrate A (i.e. magnesium nitrate solution ), the filter residue A is washed to obtain raw gypsum, and the filtrate A enters the circulation process. The steps of the circulation process are: 1) the preparation of magnesium hydroxide: adding the organic amine to the filtrate A, stirring evenly, and the temperature is 20 DEG C. React for 0.5 hours, then filter to obtain filter residue B (i....
Embodiment 2
[0034] The raw materials used in the present invention have a. 1 part of calcium nitrate, which is 1 mol; b. 1 part of organic amine, which is di-n-pentylamine, which is 2.4 mol; c. 1 part of calcium oxide-containing raw material, which is effective before use Calcium oxide content is 1.2mol; d. 2 parts of magnesium sulfate raw material, the magnesium sulfate content is measured before use, each part is 1mol.
[0035] The starting step of the process cycle of the present invention is firstly preparing calcium nitrate into a 0.1mol / L solution, then slowly adding the magnesium sulfate raw material into the solution, and then filtering to obtain filter residue A (i.e. raw gypsum) and filtrate A (i.e. magnesium nitrate solution), the filter residue A is washed to obtain raw gypsum, and the filtrate A enters the circulation process. The steps of the circulation process are: 1) the preparation of magnesium hydroxide: adding the organic amine to the filtrate A, stirring evenly, at a t...
Embodiment 3
[0038] The raw materials used in the present invention have a. 1 part of calcium nitrate, which is 1 mol; b. 1 part of organic amine, which is n-hexylamine, which is 2.2 mol; The content is 1.5 mol; d. 2 parts of magnesium sulfate raw material, the content of magnesium sulfate in it is measured before use, and each part is 1 mol.
[0039]The start-up step of the process cycle of the present invention is firstly preparing calcium nitrate into a 1.5mol / L solution, then slowly adding the magnesium sulfate raw material into the solution, and then filtering to obtain filter residue A (i.e. raw gypsum) and filtrate A (i.e. magnesium nitrate solution), the filter residue A is washed to obtain raw gypsum, and the filtrate A enters the circulation process. The steps of the circulation process are: 1) Preparation of magnesium hydroxide: adding organic amines to the filtrate A, stirring evenly, at a temperature of 50 ° C React for 0.5 hours, then filter to obtain filter residue B (i.e. m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com