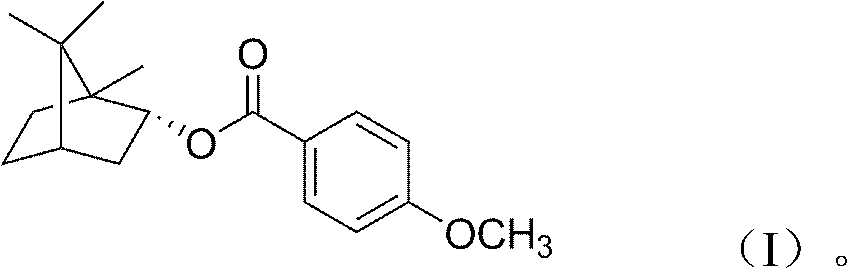
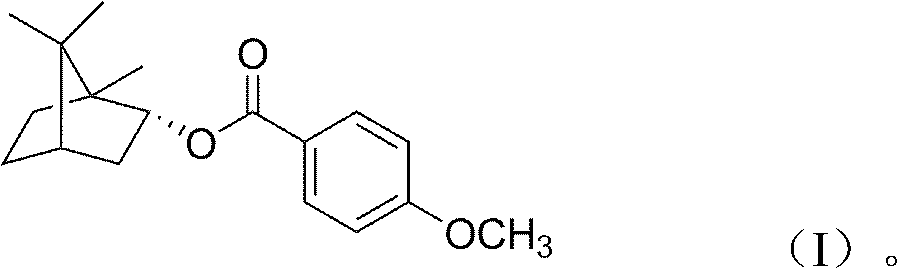
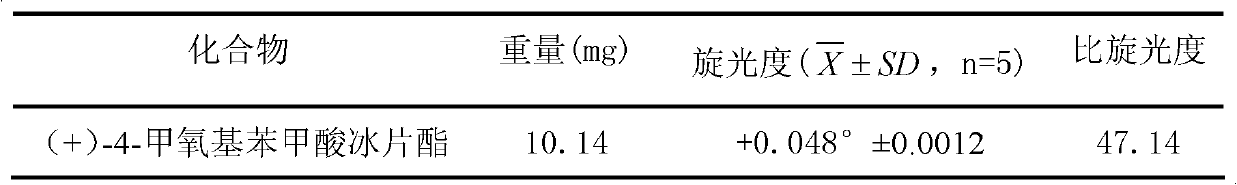
(+)-4-methoxybornyl benzoate and its preparation method and application
A technology of methoxybenzoic acid and p-toluenesulfonic acid, which is applied in the field of benzoic acid esters, can solve the problems of unsatisfactory ability of borneol to penetrate the blood-brain barrier and limit applications, and achieve the goal of promoting the penetration of blood-brain barrier drugs , low cytotoxicity, and good effect of penetrating the blood-brain barrier
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] 1. Synthesis of (+)-4-methoxybornyl benzoate
[0014] Add 20g of natural borneol, 15g of 4-methoxybenzoic acid and 30ml of toluene into a 500ml round bottom flask; add 0.5g of p-toluenesulfonic acid dropwise, mix well; stir and reflux at 120°C for 8 hours. After the reaction, the catalyst, unreacted borneol, and solvent were removed to obtain the initial product. The primary product was separated with a silica gel column to obtain colorless crystals.
[0015] 2. Properties of (+)-4-methoxybornyl benzoate
[0016] The colorless crystals obtained in step 1 are poorly soluble in water, but easily soluble in chloroform, ethanol, acetone, and ethyl acetate. The melting point is 66.3-68.6°C.
[0017] 3. Identification of the compounds of the present invention:
[0018] 1. UV spectrum analysis: the compound described in the invention is dissolved in methanol, and then scanned with a PDA-100 diode array detector (DAD), and the compound has ultraviolet absorption at 212.3nm ...
Embodiment 2
[0035] 1. Acute toxicity test of dextroborneol in mice
[0036] 1.1. Experimental animals
[0037] 60 Kunming white mice, half male and half male, body weight 20±2g. Provided by the Guangdong Provincial Laboratory Animal Center, certificate number: SCXK Yue 2008-0001 Yue Jian Zheng Zi 2007A013, used for the test after 3 days of adaptation.
[0038] 1.2. Experimental method
[0039] Mice were fasted overnight for 15 h before administration, and had free access to water. They were divided into 6 groups with 10 rats in each group. Groups 1 to 5 were given dextroborneol blended oil solution, and the dosages were 6170, 4319, 3023, 2116, 1481 mg / kg; Blend oil. According to the weight of the mice, the drug group was fed with dextroborneol at a dose of 0.2 mL / 10 g, and the control group was fed with the same volume of edible oil. Return to normal feeding 2 hours after administration, observe continuously for 14 days, observe the changes in the weight of the mice every day, whethe...
Embodiment 3
[0063] Experimental Study of (+)-4-Methoxybornyl Benzoate Penetrating the Blood Brain Barrier
[0064] 1. Test animals: SPF grade Kunming mice, half male and half male, weighing 20±2g. Provided by the Experimental Animal Center of the Guangdong Provincial Department of Health, certificate number: SCXK Yue 2008-0002, used for experiments after 3 days of adaptation. Kunming mice were fasted overnight for 15 hours before administration, and had free access to water.
[0065] 2. Method
[0066] 2.1 Liquid preparation
[0067] Accurately weigh 40mg of Evans blue and transfer it into a 10ml volumetric flask, add normal saline to the volume to obtain a 0.4% solution; mix acetone and normal saline (7:3) (v:v) to obtain a homogeneous solution slurry mixture.
[0068] 2.2 Evansland standard curve drawing
[0069] Prepare Evans blue multiple release series concentration solution 0.5ug / g, 10ug / g, 20ug / g, 50ug / g, 100ug / g with blank mouse brain plasma, and measure the absorbance at 620...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Ld50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com