Quinoxaline unit-containing fluorene copolymer, and preparation method and application thereof
A kind of copolymer and unit fluorene technology, which is applied in the field of fluorene-based copolymer containing quinoxaline unit and its preparation, can solve the mismatch between the spectral response of the device and the solar radiation spectrum, the low collection efficiency of the carrier electrode, and the lack of the red light region. Effective utilization and other issues, to achieve the effect of simple preparation route, high yield, easy operation and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
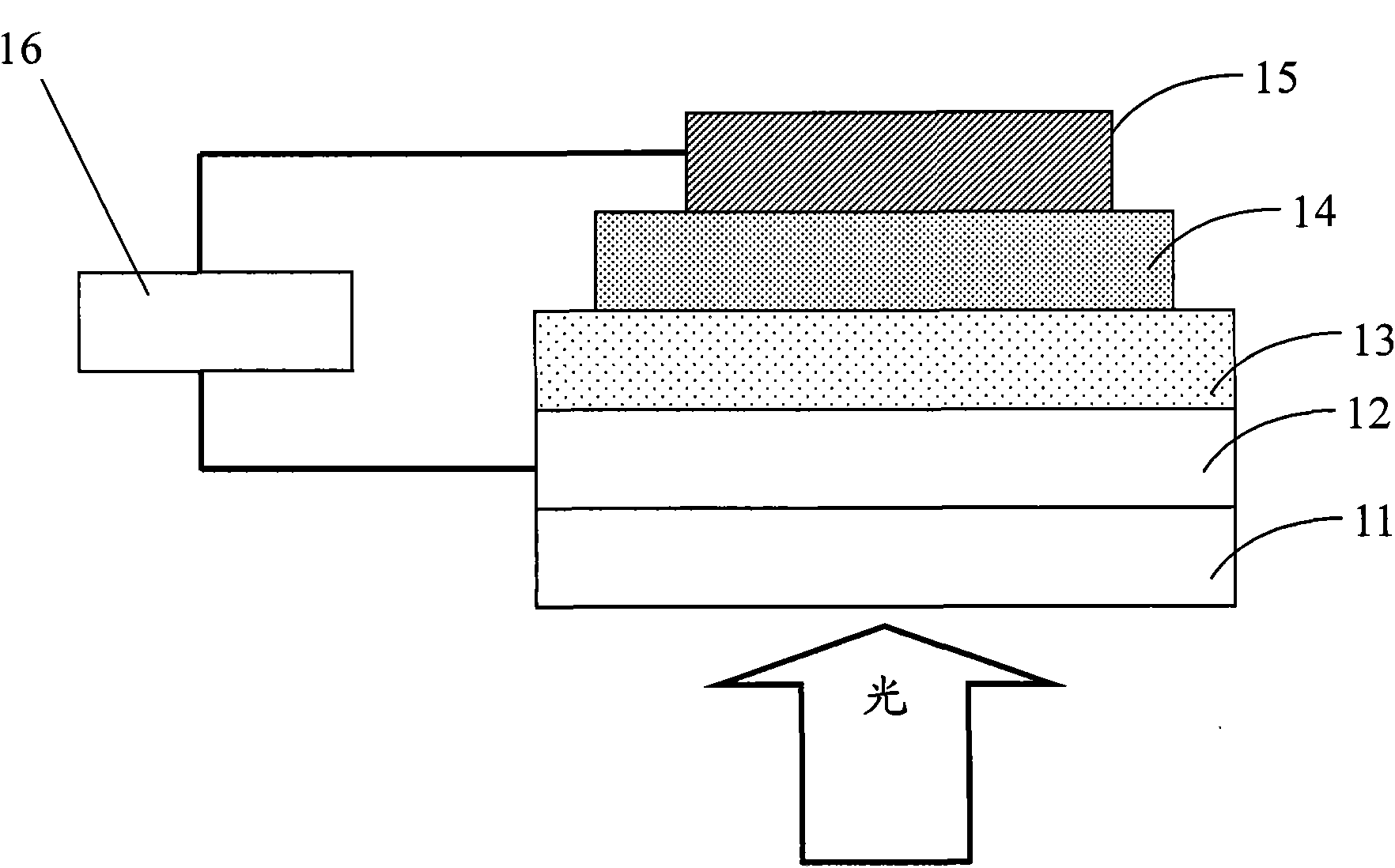
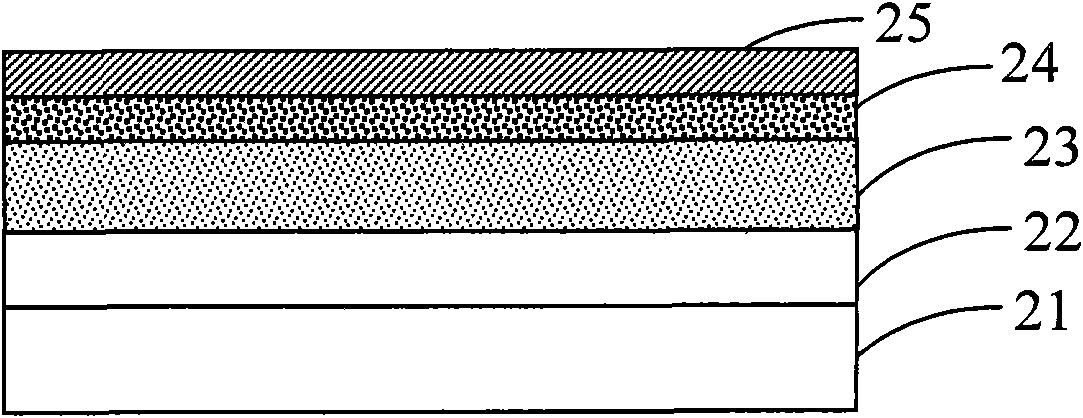
Image
Examples
preparation example Construction
[0035] And, the embodiment of the present invention also provides the preparation method of the fluorene copolymer containing quinoxaline unit, comprising the following steps:
[0036] 1) Provide compounds A, B, and C represented by the following structural formula respectively,
[0037]
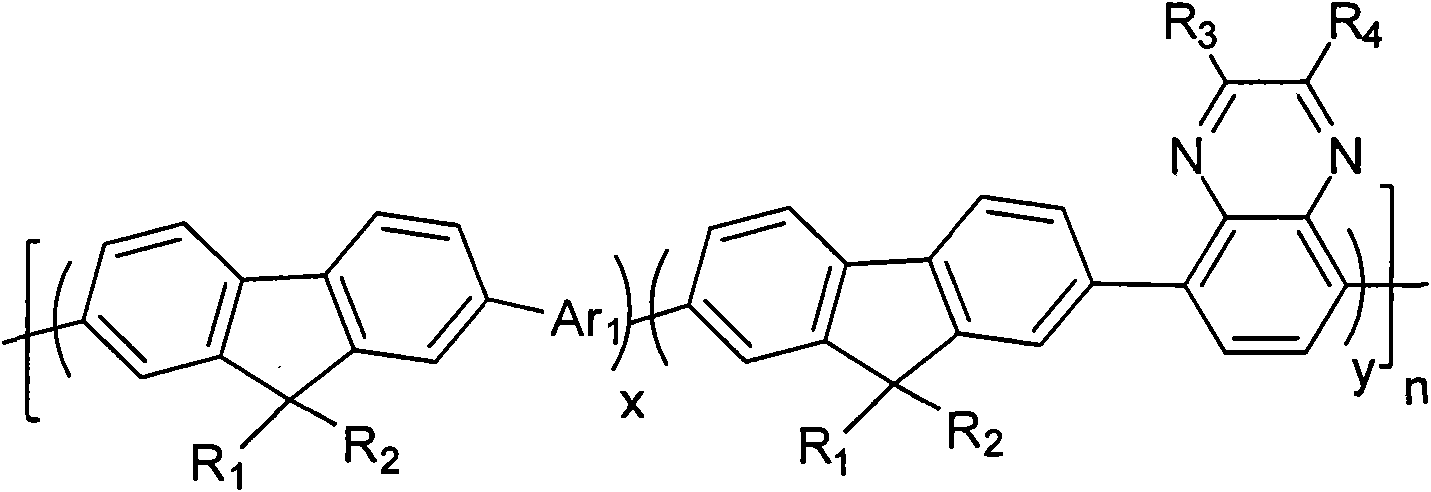
[0038] Among them, R 1 , R 2 Choose from H or C 1 ~C 20 the alkyl group; R 3 , R 4 from C 1 ~C 20 Alkyl, alkoxy, phenyl or phenoxy; Ar 1 It is a group containing a thiophene unit;
[0039]2) Under the conditions of anaerobic, alkaline environment and the presence of catalyst and organic solvent, compound A, B and C are subjected to Suzuki coupling reaction to obtain the quinoxaline unit-containing fluorene represented by general formula (I) class copolymer,
[0040]
[0041] In the formula, x+y=1, and x≠0, y≠0; n=any integer from 1 to 200,
[0042] The chemical reaction formula of this Suzuki coupling reaction is as follows:
[0043]
[0044] In the above-mentioned step ...
Embodiment 1
[0062] The copolymer of present embodiment 1 is the fluorene-based copolymer I containing quinoxaline unit represented by the following molecular structural formula 1 , its structural formula is as follows:
[0063]
[0064] Its preparation steps are as follows:
[0065] (1) Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dimethylfluorene, whose structural formula is as follows :
[0066]
[0067] The specific process of preparation is: at -100°C, under the condition of anhydrous and nitrogen protection, add 20.00mL of n-butyllithium oil solution with a concentration of 1.00M to 3.52g of 2,7-dibromo-9,9 - In a reaction flask of dimethylfluorene and 100mL tetrahydrofuran, after stirring for 2 hours, slowly add 4.17mL 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-di Oxaborane, returned to room temperature, continued to stir for 24 hours, after the reaction was completed, the reaction solution was poured into water, extracted with ether, dried over anhydrous ...
Embodiment 2
[0078] The copolymer of present embodiment 2 is the fluorene-based copolymer I containing quinoxaline unit represented by the following molecular structural formula 2 The preparation, its structural formula is as follows:
[0079]
[0080] Its preparation steps are as follows:
[0081] (1) Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dimethylfluorene, whose structural formula is as follows , see Example 1 for the preparation steps.
[0082]
[0083] (2) The preparation of 3,6-dimethylthieno[3,2-b]thiophene, its structural formula is as follows:
[0084]
[0085] The specific process of preparation is: 12.00g 3,6-dibromo-thieno[3,2-b]thiophene and 132mg (1,1'-bis(diphenylphosphino)ferrocene)palladium chloride ( Ⅱ) Add it to a 100mL tubular glass bottle equipped with a stirring rod, seal it, blow it with nitrogen, add 30mL tetrahydrofuran and 50mL methyl zinc bromide (dissolve methyl zinc bromide in tetrahydrofuran solution, its concentration is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com