Application of Ethoxysanguinarine in Pharmaceuticals
The technology of ethoxysanguinarine and medicinal salt is applied in the application field of ethoxysanguinarine in pharmaceuticals, and achieves the effects of convenient medication, small toxic and side effects, and moderate dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] When the Marc-145 cells on the 96-well cell plate (Corning Company) grew to a dense monolayer, the compound monomer ethoxysanguinarine EOSN (obtained by crystallization of sanguinarine in ethanol) was prepared with DMEM culture medium. 2-fold serial dilution, 5 gradients were serially diluted; 3 cell wells were inoculated in each gradient, the total amount of the mixed solution was 100 μl, and normal cell control was set at the same time; after 3 days of culture in a carbon dioxide incubator at 37°C, add 2-(2- CCK of methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfonic acid phenyl)-2H-tetrazole monosodium salt (WST-8) -8 Detection reagent, incubate for another 4 hours, then measure its absorbance at 450nm (OD450) with a microplate reader, and use the Reed-Much method to obtain the half-toxicity concentration TC of EOSN to cells according to the OD value 50 (50% toxic concentration) and the maximum non-toxic dose of TC 0 (0% toxic concentration) values were 53.1...
Embodiment 2
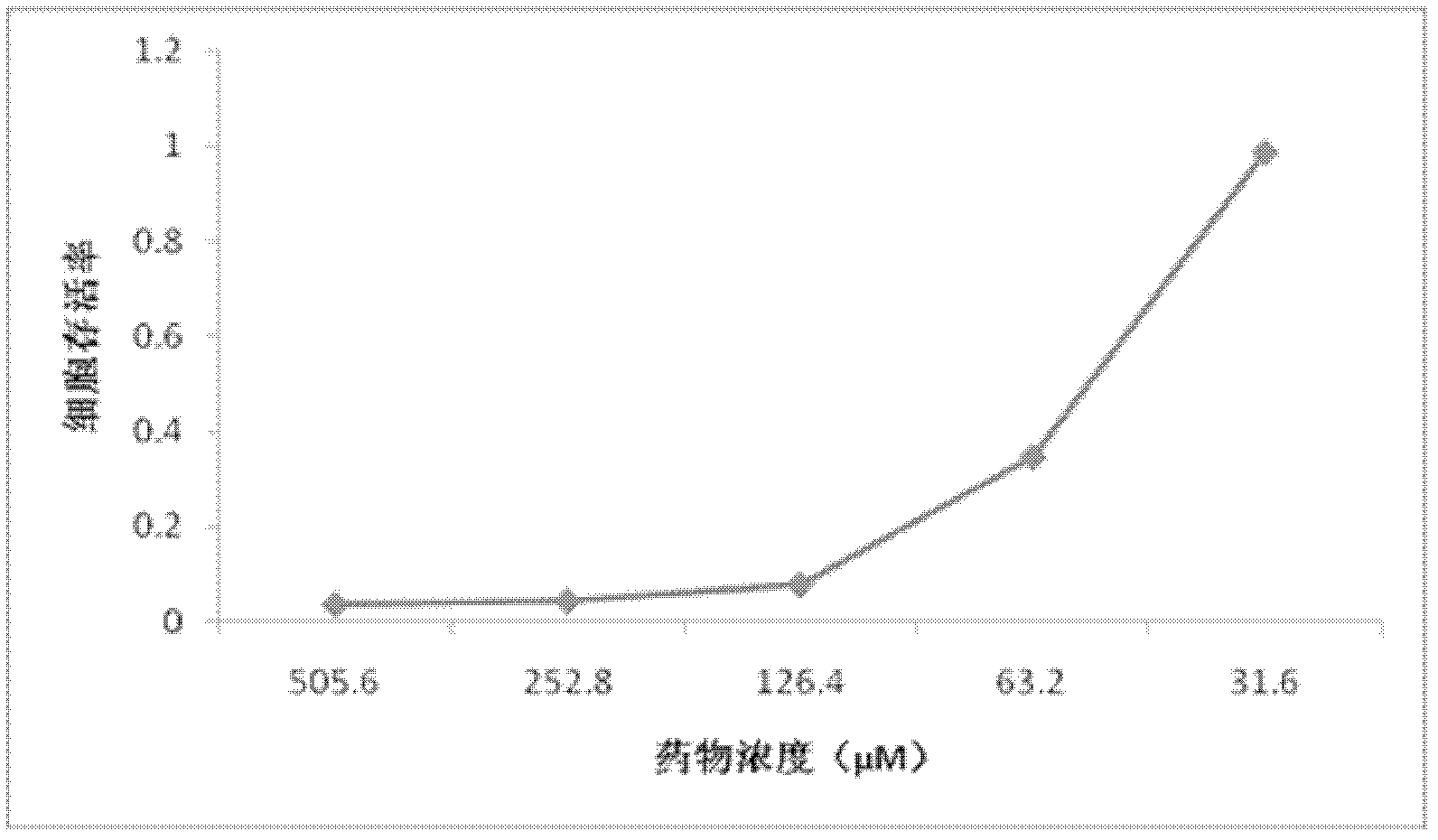
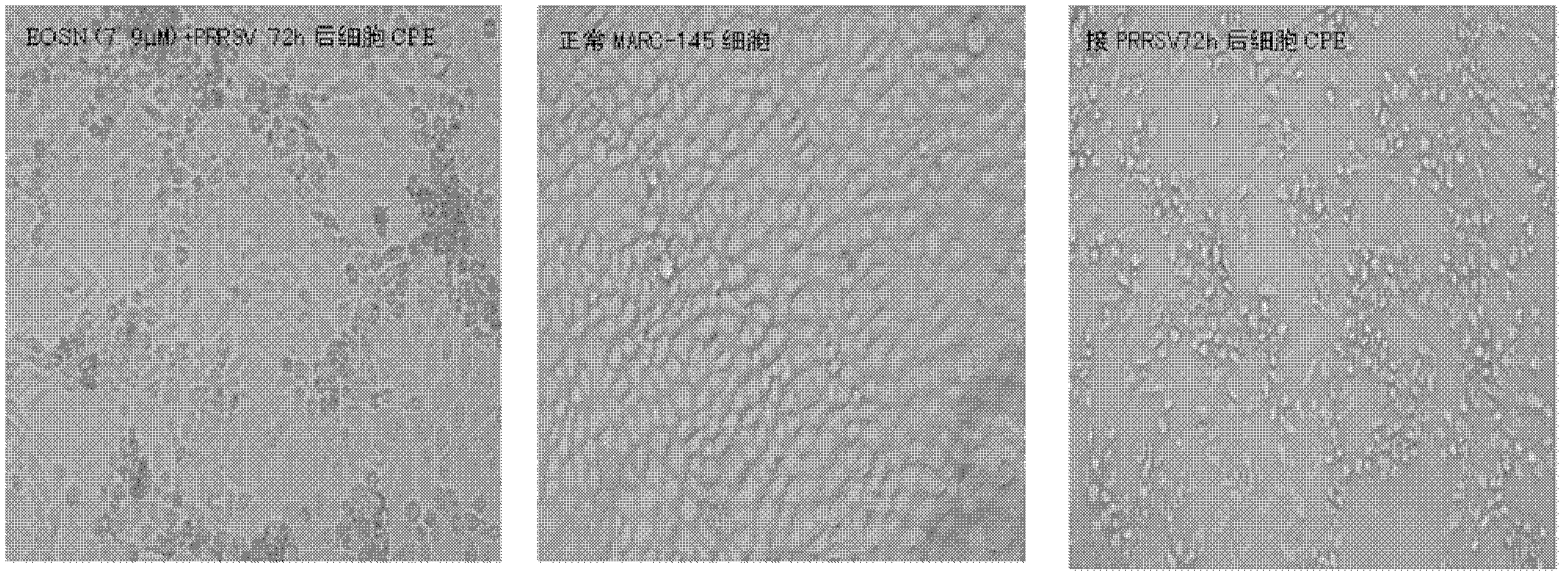
[0030] Inoculate Macr-145 cells in a 96-well cell plate to make it cover a single layer; make 2-fold serial dilutions of EOSN, and then mix different concentrations of extracts with 300 TCID 50The virus solution is mixed, and then the virus drug mixture is added to the cells. At the same time, a normal cell control group (without drug and virus) and a virus control group (only containing virus) are set up, and the culture is continued in a 5% CO2 incubator at 37°C. Daily observation of viral CPE (cytopathic effect), the CPE of Marc-145 cells caused by PRRSV mainly caused cells to shrink, aggregate, and finally fall off; after 72 hours of culture, the difference in CPE changes in different treatment groups suggested that EOSN was in the concentration range of 7.9-31.6μM It can inhibit the proliferation of PRRS virus in Marc-145 cells, significantly reduce the damage effect (CPE) of the virus on cells, and the effect is dose-dependent ( figure 2 ); according to the CPE changes ...
Embodiment 3
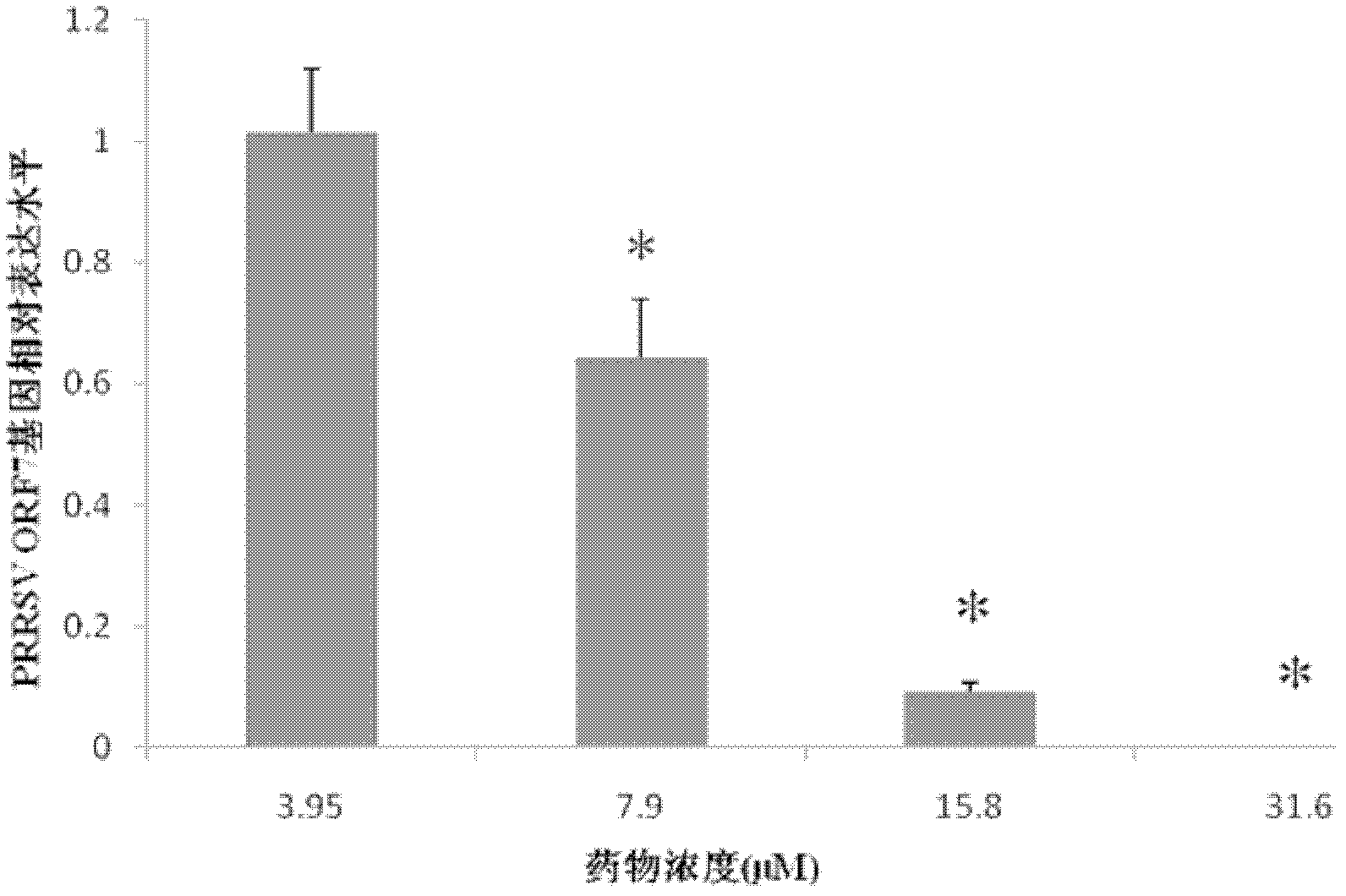
[0032] The real-time RT-PCR method was used to detect the effect of drugs on the expression of PRRSV ORF7 gene mRNA, the RNA extraction reagent RNAisoTMPlus was used to extract the total RNA of the drug group and the virus control group, and the extracted total RNA was extracted using the iQ5 real time PCR instrument (Bio-Rad ) to carry out purpose amplification, according to the β-action gene and the CT value of PRRSV ORF7 gene obtained by fluorescence quantification, use the Pfaffl method to calculate the expression ratio of the ORF7 gene of the experimental group relative to the virus control group. The results showed that: when the compound EOSN concentrations were 7.9, 15.8, and 31.6 μM, the expression of ORF7 mRNA of PRRS virus was significantly lower than that of the virus control group (P image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com