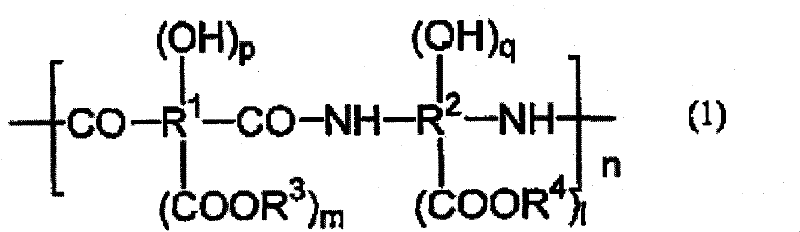
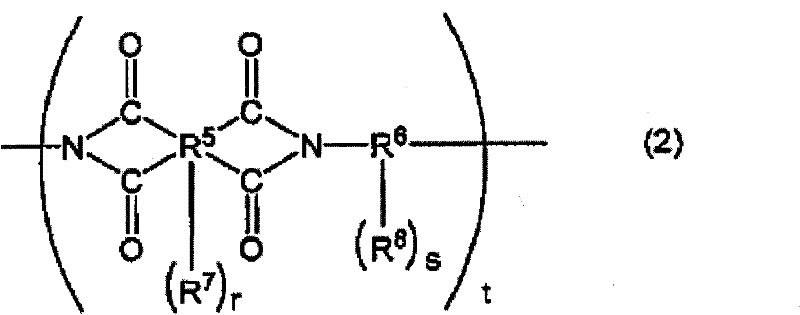
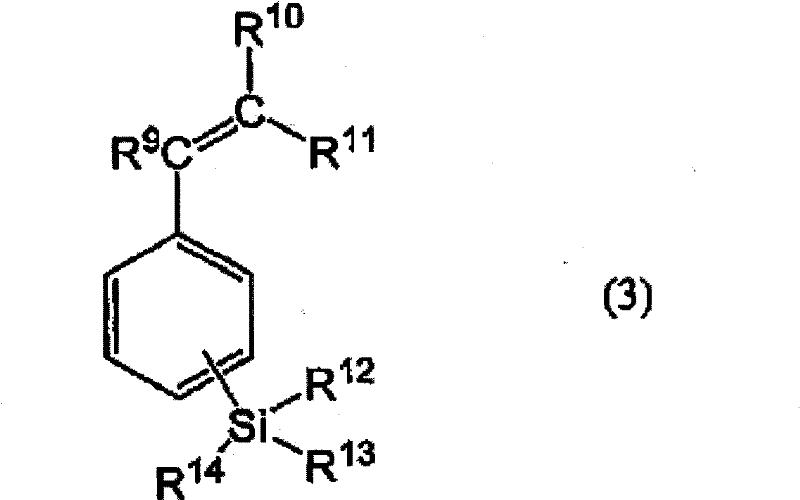
Positive-type photosensitive resin composition
A technology of photosensitive resin and composition, applied in optics, optomechanical equipment, instruments, etc., can solve the problems of insufficient storage stability and low sensitivity, and achieve the effect of excellent sensitivity stability and excellent adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0092] The present invention will be described below with reference to Examples and the like, but the present invention is not limited to these examples. In addition, the evaluation of the positive photosensitive resin composition in an Example was performed by the following method.
[0093] (1) Evaluation of storage stability of sensitivity
[0094] Preparation of photosensitive resin film
[0095] A positive-type photosensitive resin composition (hereinafter referred to as varnish) was coated on a 6-inch silicon wafer so that the film thickness after prebaking was 8 μm, and then a hot plate (mark-developer Mark-Tokyo Electron Co., Ltd.) was used 7) Prebake at 120° C. for 3 minutes to obtain a photosensitive resin film.
[0096] How to measure film thickness
[0097] Using Lambda Ace STM-602 manufactured by Dainippon SCREEN Co., Ltd., the film after prebaking and development was measured with a refractive index of 1.629, and the cured film with a refractive index of 1.773....
Synthetic example 1
[0110] Synthesis Example 1 Synthesis of an acid anhydride (a) having a hydroxyl group
[0111] Under a stream of dry nitrogen, 18.3 g (0.05 moles) of 2,2-bis(3-amino-4-hydroxyphenyl)hexafluoropropane (BAHF) and 34.2 g (0.3 moles) of allyl glycidyl ether were dissolved in 100 g of γ-butyrolactone (GBL), cooled to -15°C. 22.1 g (0.11 mol) of trimellitic anhydride acid chloride dissolved in 50 g of GBL was added dropwise so that the temperature of the reaction liquid did not exceed 0°C. After completion of the dropwise addition, the reaction was carried out at 0°C for 4 hours. The solution was concentrated with a rotary evaporator, and poured into 1 L of toluene to obtain an acid anhydride (a) having a hydroxyl group represented by the following formula.
[0112]
Synthetic example 2
[0113] Synthesis Example 2 Synthesis of diamine compound (b) having hydroxyl group
[0114] 18.3 g (0.05 mol) of BAHF were dissolved in 100 mL of acetone and 17.4 g (0.3 mol) of propylene oxide, and cooled to -15°C. Thereto, 20.4 g (0.11 mol) of 3-nitrobenzoyl chloride dissolved in 100 mL of acetone was added dropwise. After completion of the dropwise addition, the reaction was carried out at -15°C for 4 hours, and then returned to room temperature. The precipitated white solid was filtered, and vacuum-dried at 50 degreeC.
[0115] 30 g of the solid was placed in a 300 mL stainless steel autoclave, dispersed in 250 mL of methyl cellosolve, and 2 g of 5% palladium-carbon was added. Hydrogen was introduced into it with a balloon, and the reduction reaction was carried out at room temperature. After about 2 hours, the reaction was terminated after confirming that the balloon was no longer deflated. After the completion of the reaction, the palladium compound serving as a cata...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com