A kind of carbon gel catalyst and its application
A catalyst, carbon gel technology, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of low activity and poor stability, and achieve high catalytic activity, high stability, high catalytic activity. The effect of specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
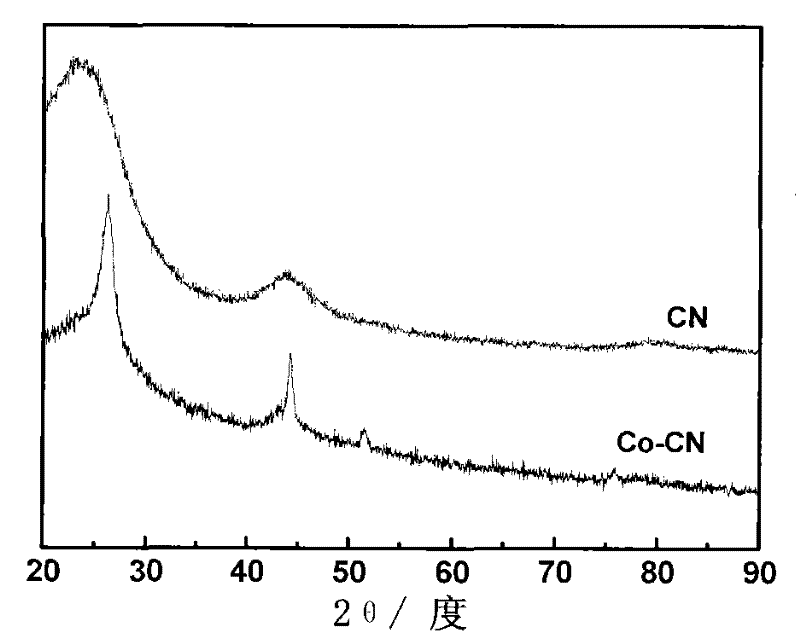
[0033] Dissolve 6.16g of resorcinol in 7mL of deionized water to form a transparent solution A; take 0.4355g of cobalt acetate tetrahydrate solid and add it to the above transparent solution A while stirring, and mix well to obtain solution B; Add dropwise 9.08g of formaldehyde solution with a mass concentration of 37%, stir and mix evenly, add dropwise 3mL of ammonia water with a mass concentration of 28% in an environment of 20°C and continue to seal and stir, the reaction forms a gel C; transfer the gel C to a vacuum Vacuum drying and aging treatment at 60°C in a drying oven for 3 days, crushing and grinding after taking out to obtain solid powder D; carbonitriding treatment of solid powder D at 900°C in an acetonitrile atmosphere for 1 hour, N 2 Gas purged to room temperature, 2M HNO 3 The solution was washed to remove metal salts to obtain a carbon xerogel catalyst Co-CN-32 with a molar ratio of resorcinol and cobalt acetate of 32:1.
Embodiment 2
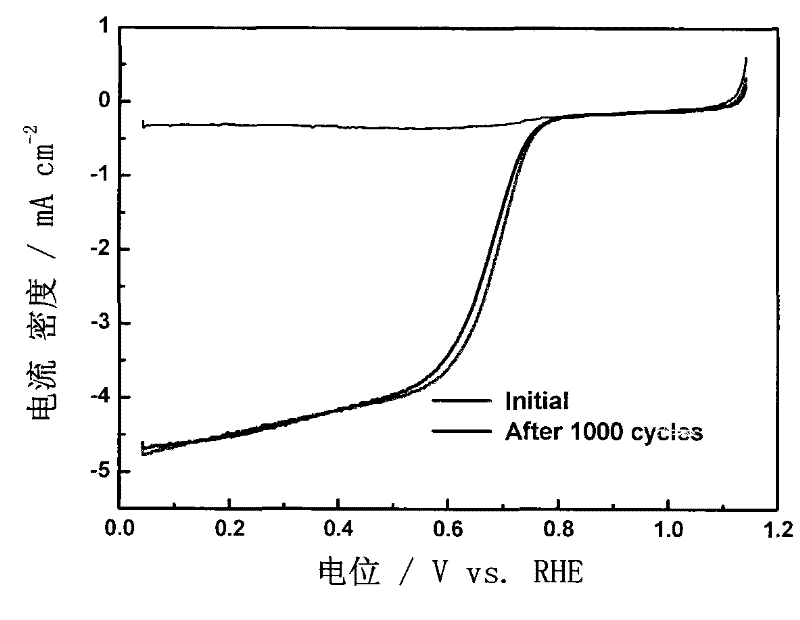
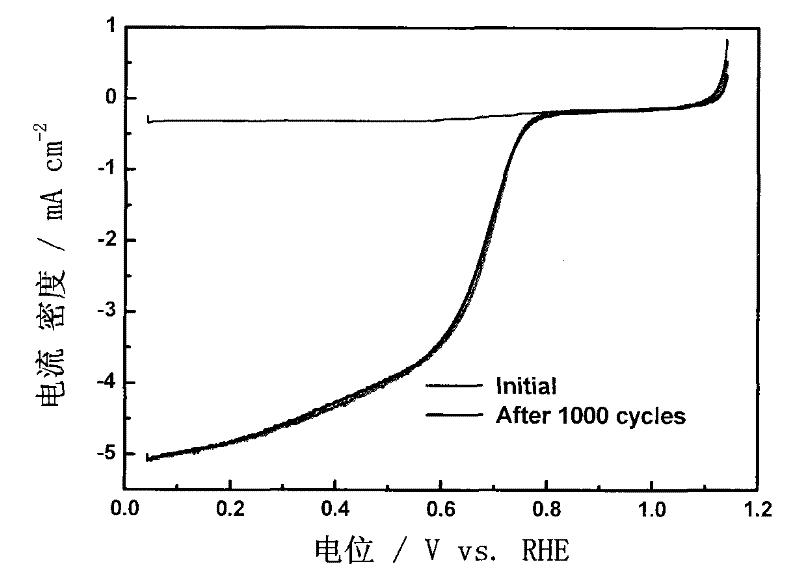
[0040] Dissolve 6.16g of resorcinol in 7mL of deionized water to form a transparent solution A; weigh 0.8149g of cobalt nitrate hexahydrate and add it into the above-mentioned transparent solution A while stirring, mix and dissolve evenly to obtain a solution B; 9.08g of formaldehyde solution with a mass concentration of 37% was added dropwise to the mixture, further stirred and mixed evenly, and 7mL of ammonia water with a mass concentration of 20% was added dropwise at the same time in an environment of 20°C and continuously sealed and stirred, and the reaction formed gel C; C was dried and aged in supercritical CO2 for 7d, and after taking it out, it was pulverized and ground to obtain solid powder D; 3 Carbonitriding treatment at 800°C for 3h, N 2 Gas purged to room temperature, 0.5M H 2 SO 4 The solution was washed to remove metal salts to obtain a carbon airgel catalyst Co-CN-20 with a molar ratio of resorcinol and cobalt nitrate of 20:1.
[0041] image 3 It can be ...
Embodiment 3
[0043] Dissolve 6.16g of resorcinol in 2mL of deionized water to form a transparent solution A; take 0.7063g of ferric nitrate nonahydrate solid and add it to the above transparent solution A while stirring, mix and dissolve evenly to obtain solution B; Add dropwise 9.08g of formaldehyde solution with a mass concentration of 37% in the solution, further stir and mix evenly, continue stirring in an environment of 90°C, and form gel C after 3 hours of reaction; transfer gel C to a vacuum drying oven for vacuum drying at 80°C Aging treatment for 3d, crushing and grinding after taking out to obtain solid powder D; solid powder D in NH 3 Carbonitriding treatment at 700°C for 5 hours, purged with Ar gas to room temperature, 4M HNO 3 The solution was washed to remove metal salts to obtain a carbon xerogel catalyst Fe-CN-32 with a molar ratio of resorcinol and iron nitrate of 32:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com