Method for preparing microsphere preparation coated with hydrophilic medicaments
A technology of hydrophilic drugs and microspheres, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, block delivery, etc., and can solve the problems of inability to maintain long-term release of drugs, increase drug toxicity and side effects, and low encapsulation efficiency , to achieve controllable drug release behavior, improve hydrophilicity, and avoid burst release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
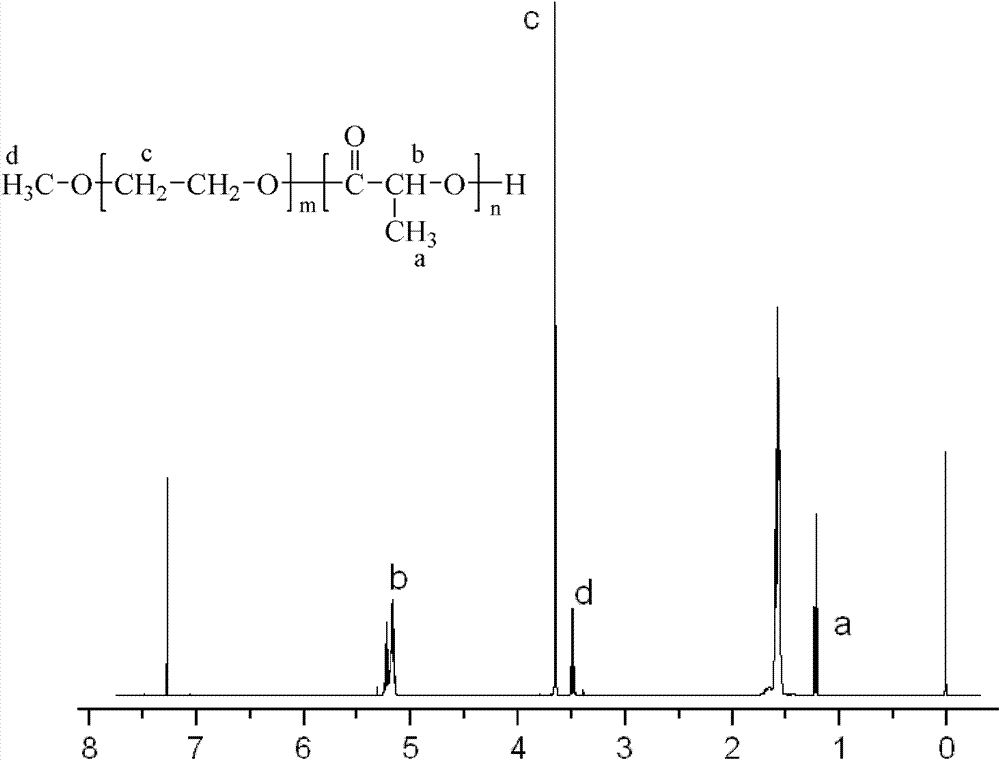
[0040] Weigh 4.5g of D, L-lactide and 0.5g of polyethylene glycol 5000 monomethyl ether and dissolve in 100ml of anhydrous toluene, add 25mg of stannous octoate as a catalyst, and reflux in an oil bath at 130°C under nitrogen protection After reacting for 24 hours, part of the toluene was distilled off under reduced pressure, the concentrated solution was poured into anhydrous ether to precipitate, the product was collected by filtration, and vacuum-dried for 24 hours to obtain a mass ratio of polylactic acid to polyethylene glycol 5000 monomethyl ether of 90:10. PLA-mPEG with a number average molecular weight of 50,000.
[0041] Weigh 20 mg of the above-mentioned PLA-mPEG, 80 mg of PLGA (the number-average molecular weight is 60,000, and the mass ratio of lactic acid to glycolic acid is 50:50) and dissolve in a mixed solvent of 0.8 ml ethyl acetate and 0.2 ml acetonitrile to prepare an organic solution. Dissolve 15 mg of leuprolide in 0.2 ml of water to prepare a hydrophilic ...
Embodiment 2
[0043] Weigh 3.5g of D, L-lactide and 1.5g of polyethylene glycol 5000 monomethyl ether and dissolve in 100ml of anhydrous toluene, add 20mg of stannous octoate as catalyst, and reflux in an oil bath at 130°C under nitrogen protection After reacting for 24 hours, part of the toluene was distilled off under reduced pressure, the concentrated solution was poured into anhydrous ether to precipitate, the product was collected by filtration, and dried in vacuum for 24 hours to obtain a mass ratio of polylactic acid to polyethylene glycol 5000 monomethyl ether of 70:30, PLA-mPEG with a number average molecular weight of 17,000.
[0044] Weigh the above-mentioned PLA-mPEG 40mg, PLGA (the number average molecular weight is 50,000, the mass ratio of lactic acid and glycolic acid is 75:25), and the mass ratio of lactic acid and glycolic acid is 75:25) 60mg is dissolved in 1.5ml ethyl acetate The mixed solvent of 0.3ml acetonitrile makes organic solution, and doxorubicin hydrochloride 20...
Embodiment 3
[0046] Weigh 3.0g of D, L-lactide and 2.0g of polyethylene glycol 5000 monomethyl ether and dissolve in 100ml of anhydrous toluene, add 20mg of stannous octoate as a catalyst, and reflux in an oil bath at 130°C under nitrogen protection After reacting for 24 hours, part of the toluene was distilled off under reduced pressure, the concentrated solution was poured into anhydrous ether to precipitate, the product was collected by filtration, and vacuum-dried for 24 hours to obtain a mass ratio of polylactic acid to polyethylene glycol 5000 monomethyl ether of 60:40. PLA-mPEG with a number average molecular weight of 13,000.
[0047] Weigh 50 mg of the above PLA-mPEG, 50 mg of PLGA (the number average molecular weight is 20,000, and the mass ratio of lactic acid to glycolic acid is 80:20) dissolved in a mixed solvent of 2 ml of ethyl acetate and 0.5 ml of acetonitrile to prepare an organic solution, rice hydrochloride Toxantrone 10mg was dissolved in 0.5ml 0.1M phosphate buffered ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com