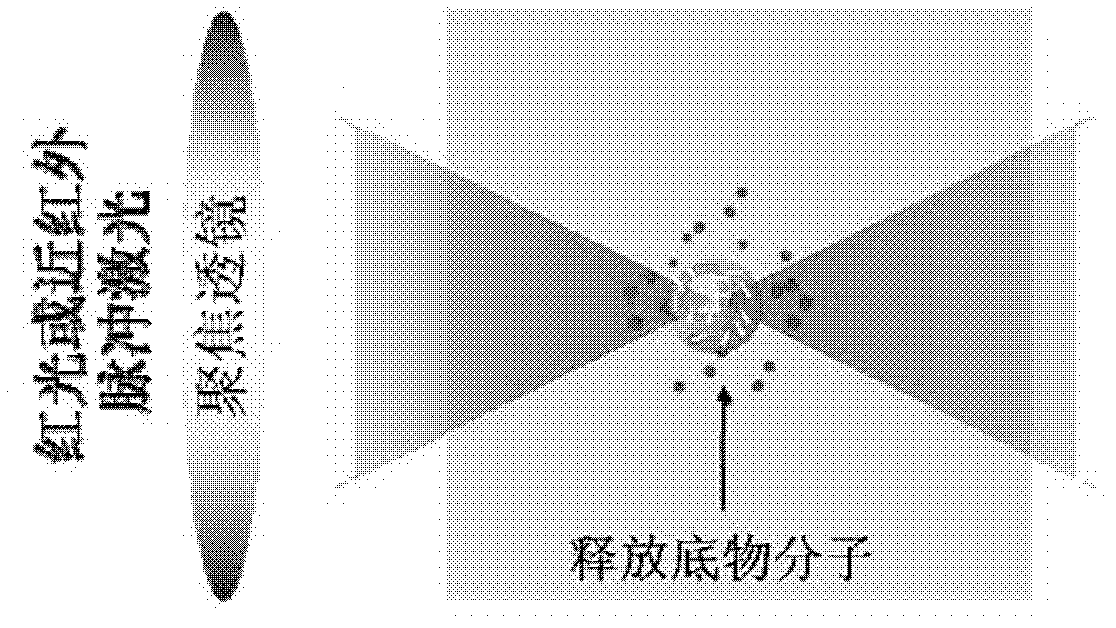
Vesicle with two-photon excitation controlled-release function, and preparation method and application thereof
A two-photon excitation and controlled release technology, which is applied in organic chemistry, liposome delivery, etc., can solve the problems of high preparation cost, low response efficiency, and small two-photon absorption cross section, and achieve good two-photon responsiveness and biophase Capacitive, repeatable, low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
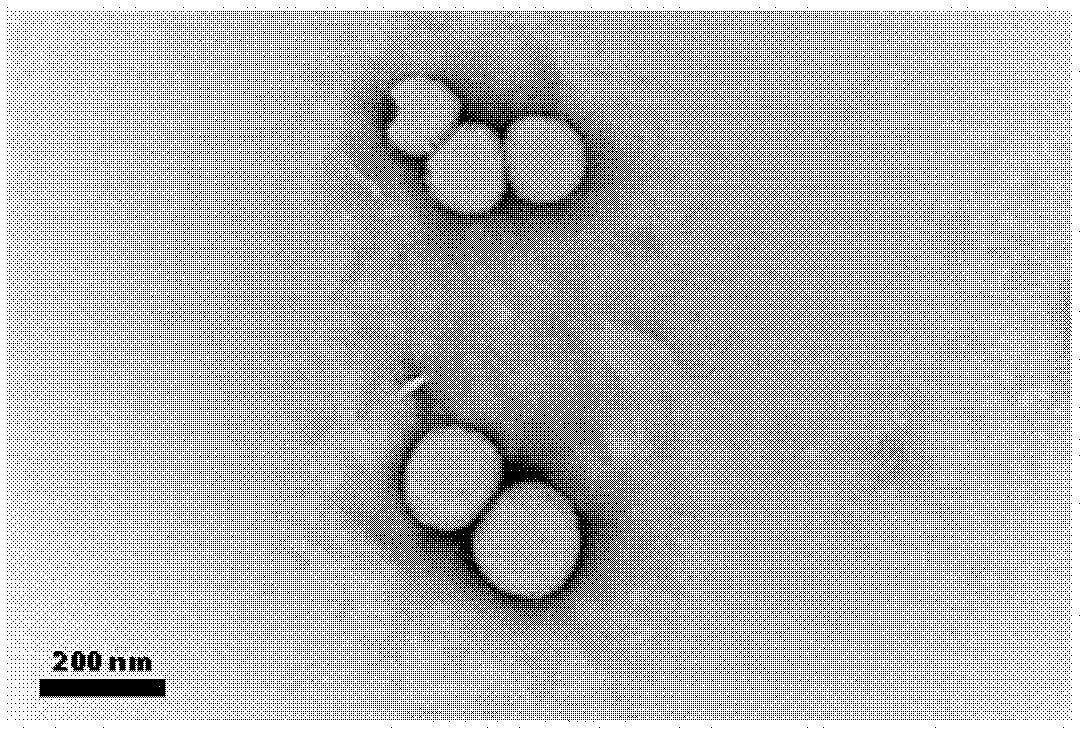
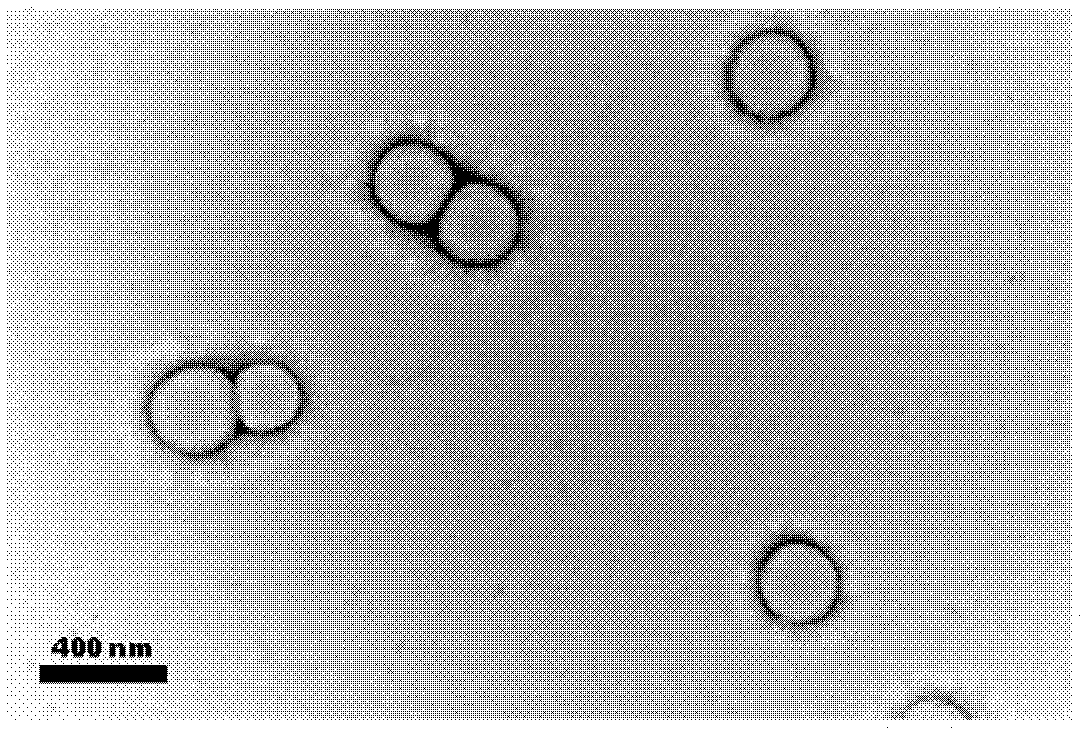
Image
Examples
Embodiment 1
[0038] The two-photon-sensitive amphiphile is prepared, and its structure is shown in formula (A).
[0039]
[0040] Formula (A)
[0041] Its reaction scheme is as follows:
[0042]
[0043] Add 1 equivalent of 4-hydroxymethyl-7-aminocoumarin, 6 equivalents of n-dodecane bromide and 8 equivalents of potassium carbonate to dry DMF to dissolve completely; reflux reaction under nitrogen protection 36 hours; after the reaction was completed, cooled, suction filtered, the filtrate was washed with water, dried, separated and purified by silica gel column chromatography to obtain a yellow solid. 1 H NMR (400MHz, CDCl 3 )δ(ppm), 7.35(d, 1H), 6.68(dd, 1H), 6.58(s, 1H), 6.30(s, 1H), 4.88(s, 2H), 3.32(t, 4H), 1.59( m, 4H), 1.31 ~ 1.28 (m, 36H), 0.89 (t, 6H). MALDI-TOF MS (m / z): [M+H] + 528.4;
[0044]Add 1 equivalent of the previously obtained compound and 1 equivalent of bromoacetic acid and a catalytic amount of 4-dimethylaminopyridine (DMAP) into dry dichloromethane to dis...
Embodiment 2
[0046] The two-photon-sensitive amphiphile is prepared, and its structure is shown in formula (B).
[0047]
[0048] Formula (B)
[0049] Its reaction scheme is as follows:
[0050]
[0051] Add 1 equivalent of 3-methyl-4-hydroxymethyl-6-chloro-7-aminocoumarin and 6 equivalents of n-dodecane bromide and 8 equivalents of potassium carbonate to dry DMF to make completely Dissolved; reflux reaction under the protection of nitrogen for 36 hours; after the reaction was completed, cooled, filtered with suction, washed the filtrate with water, dried, separated and purified by silica gel column chromatography to obtain a yellow solid product. 1 H NMR (400MHz, CDCl 3 )δ(ppm), 7.03(s, 1H), 6.71(s, 1H), 4.88(s, 2H), 3.32(t, 4H), 2.01(s, 3H), 1.59(m, 4H), 1.31~ 1.28 (m, 36H), 0.89 (t, 6H). MALDI-TOF MS (m / z): [M+H] + 576.4;
[0052] Add 1 equivalent of the previously obtained compound and 1 equivalent of bromoacetic acid and a catalytic amount of 4-dimethylaminopyridine (DMAP)...
Embodiment 3
[0054] The two-photon-sensitive amphiphile is prepared, and its structure is shown in formula (C).
[0055]
[0056] Formula (C)
[0057] Its reaction scheme is as follows:
[0058]
[0059] Add 1 equivalent of 4-hydroxymethyl-6-methoxy-7-aminocoumarin, 6 equivalents of n-dodecane bromide and 8 equivalents of potassium carbonate into dry DMF to dissolve completely; Under the protection of nitrogen, the reaction was carried out under reflux for 36 hours; after the reaction was completed, it was cooled, filtered with suction, the filtrate was washed with water, dried, separated and purified by silica gel column chromatography to obtain a yellow solid. 1 H NMR (400MHz, CDCl 3 )δ(ppm), 6.55(s, 1H), 6.49(s, 1H), 6.21(s, 1H), 4.88(s, 2H), 3.79(s, 3H), 3.32(t, 4H), 1.59( m, 4H), 1.31 ~ 1.28 (m, 36H), 0.89 (t, 6H). MALDI-TOF MS (m / z): [M+H] + 558.2;
[0060] Add 1 equivalent of the previously obtained compound and 1 equivalent of bromoacetic acid and a catalytic amount of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com