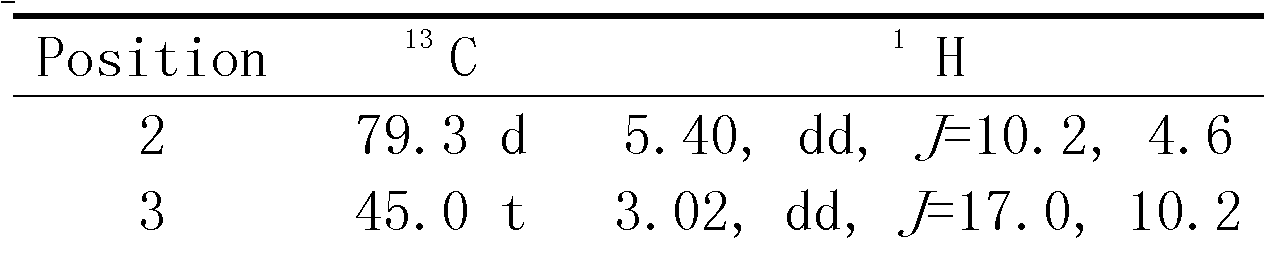Flavanone compound and application thereof
A technology of flavonoids and compounds, applied in the field of tobacco chemistry, to achieve good anti-tobacco mosaic virus activity and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Preparation of dihydroflavonoids
[0018] Tobacco rhizome samples were collected from Yuxi, Yunnan, and the variety was Honghua Dajinyuan. A sample of 1.5 kg of tobacco rhizome samples was crushed to 30 meshes, and extracted 3 times with 95% ethanol with ultrasound. The extracts were combined, filtered, and concentrated under reduced pressure to form an extract to obtain 65.8 g of extract. The extract is dissolved in analytical alcohol methanol and mixed with 100g silica gel (80-100 mesh), 1.5 kg silica gel (160-200 mesh) is packed in a column for silica gel column chromatography separation, chloroform:acetone (1:0→0:1) Gradient elution, TLC monitoring combined the same fractions to obtain 8 fractions (pure chloroform, chloroform-acetone 20:1, chloroform-acetone 9:1, chloroform-acetone 8: 2, chloroform-acetone 3: 2, chloroform-acetone 1:1, chloroform-acetone 1:2, pure acetone), of which 8.2g of chloroform-acetone (1:1) elution fraction was separated by Agilent ...
Embodiment 2
[0019] Example 2: Identification of dihydroflavonoids
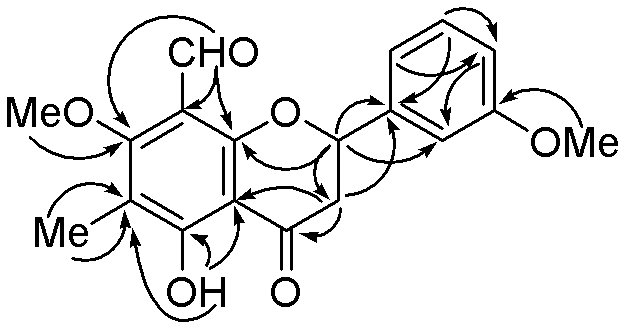
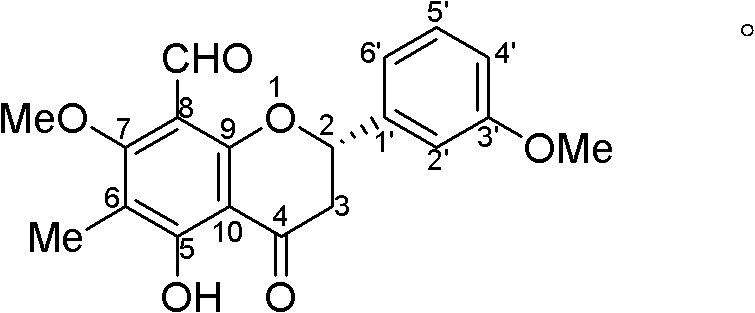
[0020] The compound of the present invention is orange-yellow powder; UV spectrum (solvent is methanol), λ max (logε) 362(3.97), 286(4.18), 268(4.26), 210(4.88) nm; infrared spectrum (potassium bromide tablet) ν max 3428, 1685, 1622, 1571, 1460cm -1 . The quasi-molecular ion peak m / z 341.1031[M-H] is given by high resolution mass spectrometry (HRESIMS) + (Calculated value 341.1025), combined 1 H and 13 C NMR spectrum gives a molecular formula C 19 H 18 O 6 , The degree of unsaturation is 11. From 1 H and 13 CNMR spectrum (see Table-1 for data attribution) signal shows that there is a dihydroflavonoid nucleus (δ C 79.3d, 45.0t, 189.5s, 164.2s, 112.4s, 165.2s, 115.6s, 163.0s, 110.2s, 139.8s, 113.2d, 162.2s, 113.8d, 130.6d, 118.5d; δ H 5.40 dd J = 10.24.6, 3.02 dd J = 17.010.2, 2.89 dd J = 4.516.8, 7.01m, 6.88 d J = 8.0, 7.22 t J = 8.0, 6.92m), 1 methyl group (δ C 8.2q, δ H 2.08s), 2 methoxy groups (δ C 55.8q, 63.6q; δ H 3.7...
Embodiment 3
[0025] Example 3: Detection of anti-tobacco mosaic virus activity of dihydroflavonoids
[0026] The conventional half-leaf method is used to test the anti-tobacco mosaic virus activity of the compound of the present invention when the mass concentration of the medicament is 50 mg / L. On the 5-6 leaf-age flue-cured tobacco plants, select the leaves suitable for testing (the leaf rows are normal, disease-free and insect-free), and the leaves are evenly sprinkled with emery, and the spare tobacco mosaic virus source (3.0× 10 -3 ) Evenly spread on the leaves sprinkled with emery. After all the selected leaves have been poisoned, put them in a petri dish containing the liquid medicine for 20 minutes, take them out, sprinkle the water drops and liquid medicine on the leaves, and combine the two The semi-leaf recovery is discharged in an enamel covered with toilet paper and covered with glass, and the temperature is controlled at (23±2) ℃. It is exposed to natural light in a greenhouse, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com