Anodic oxidation method for preparing aluminum-based super-hydrophobic film
A super-hydrophobic, water-drop technology, applied in the direction of anodic oxidation, electrolytic coating, surface reaction electrolytic coating, etc., can solve the problems of high cost and difficult industrialization, achieve good reproducibility, simple operation process, and good industrial application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
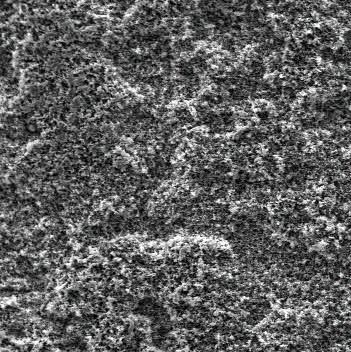
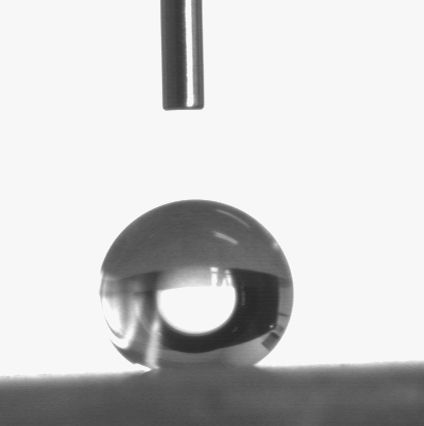
[0017] First, the aluminum alloy plate was mechanically polished and polished to remove surface defects and oxide films, and was cleaned with ethanol and deionized water for 15 minutes, respectively. The prepared electrolyte was 1.803 mol / L sulfuric acid, 0.068 mol / L potassium dichromate, 0.079 mol / L oxalic acid, 2.57 mol / L sodium chloride and 1.37 mol / L glycerin. Then use the aluminum plate as the anode and the graphite as the cathode to carry out the reaction. The control current density is 1.2 A / dm 2 , and the reaction time was 30 min. Wash the reacted aluminum sheet with distilled water and dry it or dry it naturally; soak it in stearic acid ethanol solution with a mass fraction of 1% for 30 minutes, and dry it for 24 hours at a relative humidity of 60-75% and at room temperature , the aluminum alloy porous superhydrophobic surface can be prepared. The wettability of the coating surface was tested with an OCA20 contact angle tester, and the results showed that the conta...
Embodiment 2
[0019] First, the aluminum alloy plate was mechanically polished and polished to remove surface defects and oxide films, and was cleaned with ethanol and deionized water for 15 minutes, respectively. Prepare the electrolyte as 1.37 mol / L sulfuric acid, 0.054 mol / L potassium dichromate, 0.079 mol / L oxalic acid, 2.6 mol / L sodium chloride and 1.37 mol / L glycerin. Then use the aluminum plate as the anode and the graphite as the cathode to carry out the reaction. The control current density is 1.3 A / dm 2 , and the reaction time was 35 min. Wash the reacted aluminum sheet with distilled water and dry it or dry it naturally; soak it in stearic acid ethanol solution with a mass fraction of 1% for 30 minutes, and dry it for 25 hours at a relative humidity of 60~75% and at room temperature , the aluminum alloy porous superhydrophobic surface can be prepared. The wettability of the coating surface was tested with an OCA20 contact angle tester, and the results showed that the contact ...
Embodiment 3
[0021] First, the aluminum alloy plate was mechanically polished and polished to remove surface defects and oxide films, and was cleaned with ethanol and deionized water for 15 minutes, respectively. The prepared electrolyte was 1.803 mol / L sulfuric acid, 0.06 mol / L potassium dichromate, 0.079 mol / L oxalic acid, 2.57 mol / L sodium chloride and 1.37 mol / L glycerin. Then use the aluminum plate as the anode and the graphite as the cathode to carry out the reaction. The control current density is 1.4A / dm 2 , and the reaction time was 35 min. Wash the reacted aluminum plate with distilled water and dry it or dry it naturally; soak it in stearic acid ethanol solution with a mass fraction of 1% for 30 minutes, and dry it for 26 hours at a relative humidity of 60~75% and at room temperature , the aluminum alloy porous superhydrophobic surface can be prepared. The wettability of the coating surface was tested with an OCA20 contact angle tester, and the results showed that the contact...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com