Preparation method for substituting fluorine for oxygen in bismuth ferrite crystal lattices
A bismuth ferrite, lattice technology, applied in chemical instruments and methods, iron compounds, inorganic chemistry, etc., can solve the problems of insufficient anion doping preparation process, and achieve the effect of ferromagnetism enhancement and magnetoelectric coupling.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: the content of synthetic F is the sample of 0.25 (structural formula)
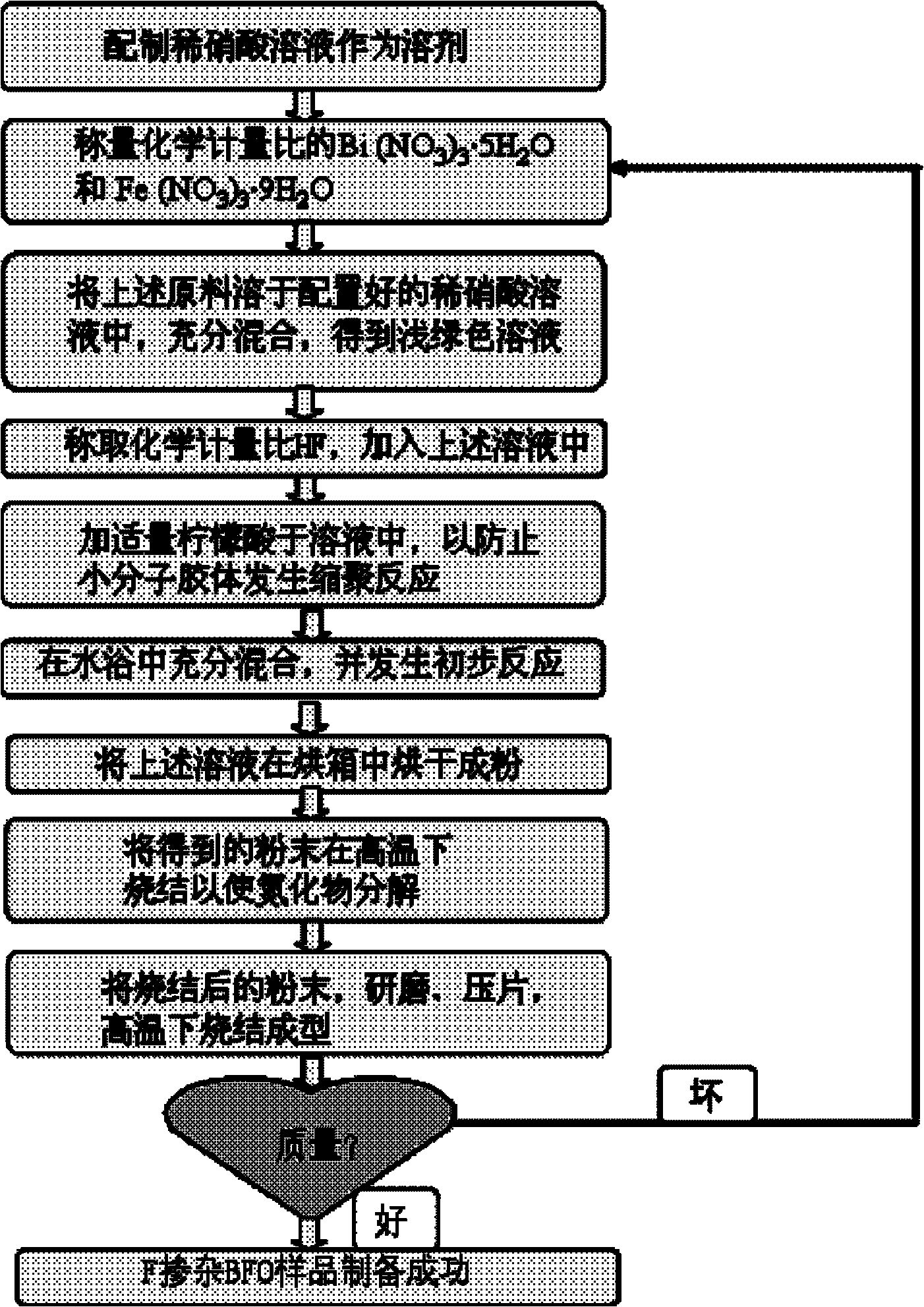
[0018] Attached figure 1 The preparation process shown, the synthesis of BiFeO with F content of 0.25 2.75 f 0.25 sample. Its synthetic steps are:
[0019] a) Use a vessel made of corrosion-resistant materials as a container. First prepare a dilute acid solution, take a 200ml beaker, and measure 100ml of the dilute acid solution. Weigh the Bi salt (Bi(NO 3 ) 3 ·5H 2 O) and Fe salts (Fe(NO 3 ) 3 9H 2 O) Add it into the dilute acid solution, stir well to make it dissolve completely, and mix evenly.
[0020] b) One beaker, one drip irrigation, one AL104 Mettler Toledo electronic balance. Weigh the stoichiometric F-containing salt (HF), add it into the solution prepared in a), and stir well to make it evenly mixed.
[0021] c) Weigh an appropriate amount of citric acid and add it to the solution prepared in b), stir fully to dissolve it completely, and mix evenly.
[0022] d) ...
Embodiment 2
[0028] Embodiment 2: the content of synthetic F is the sample of 0.2 (structural formula)
[0029] Attached figure 1 The preparation process shown, the synthesis of BiFeO with F doping amount of 0.2 2.8 f 0.2 sample. Its synthesis steps are the same as those in Example 1.
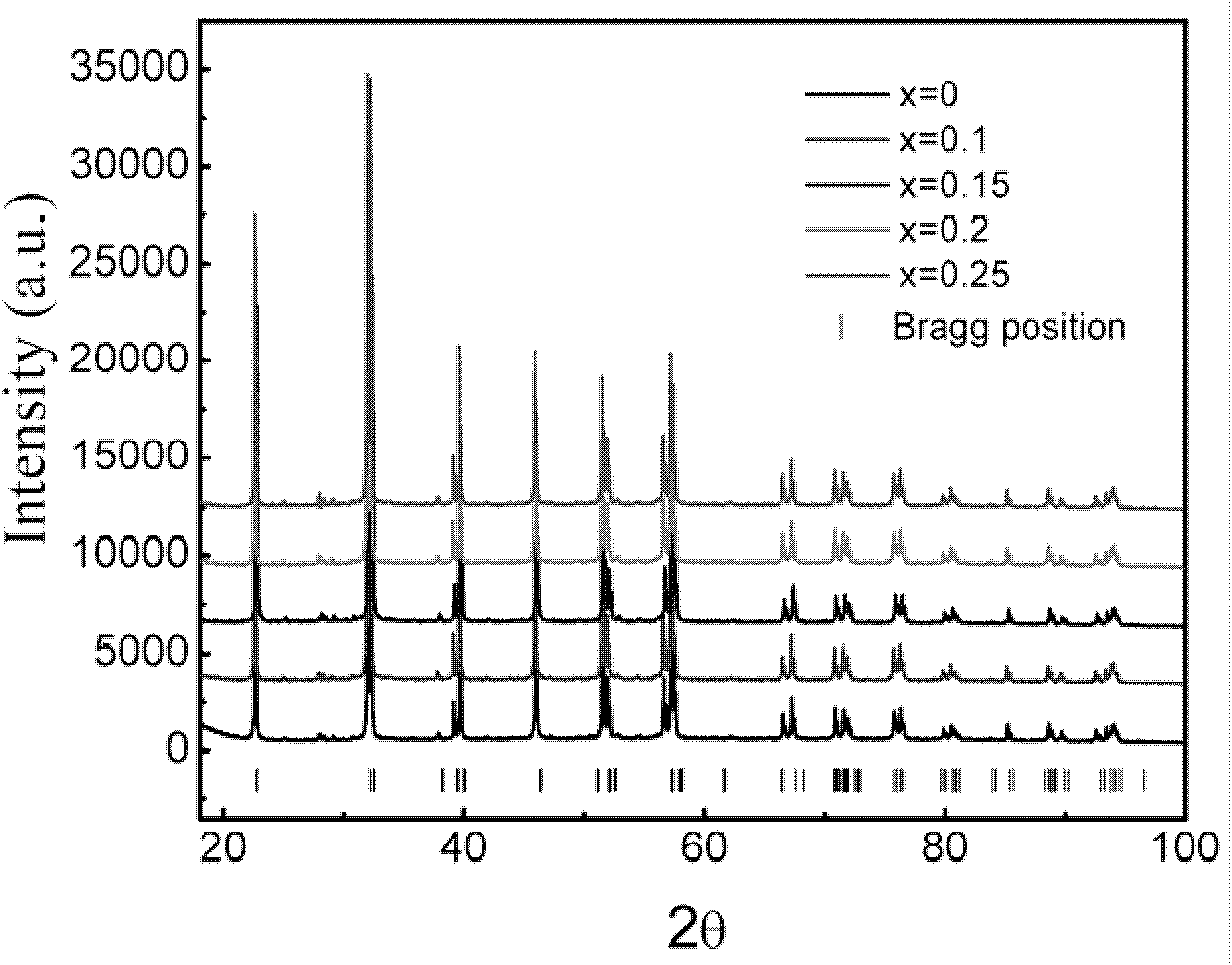
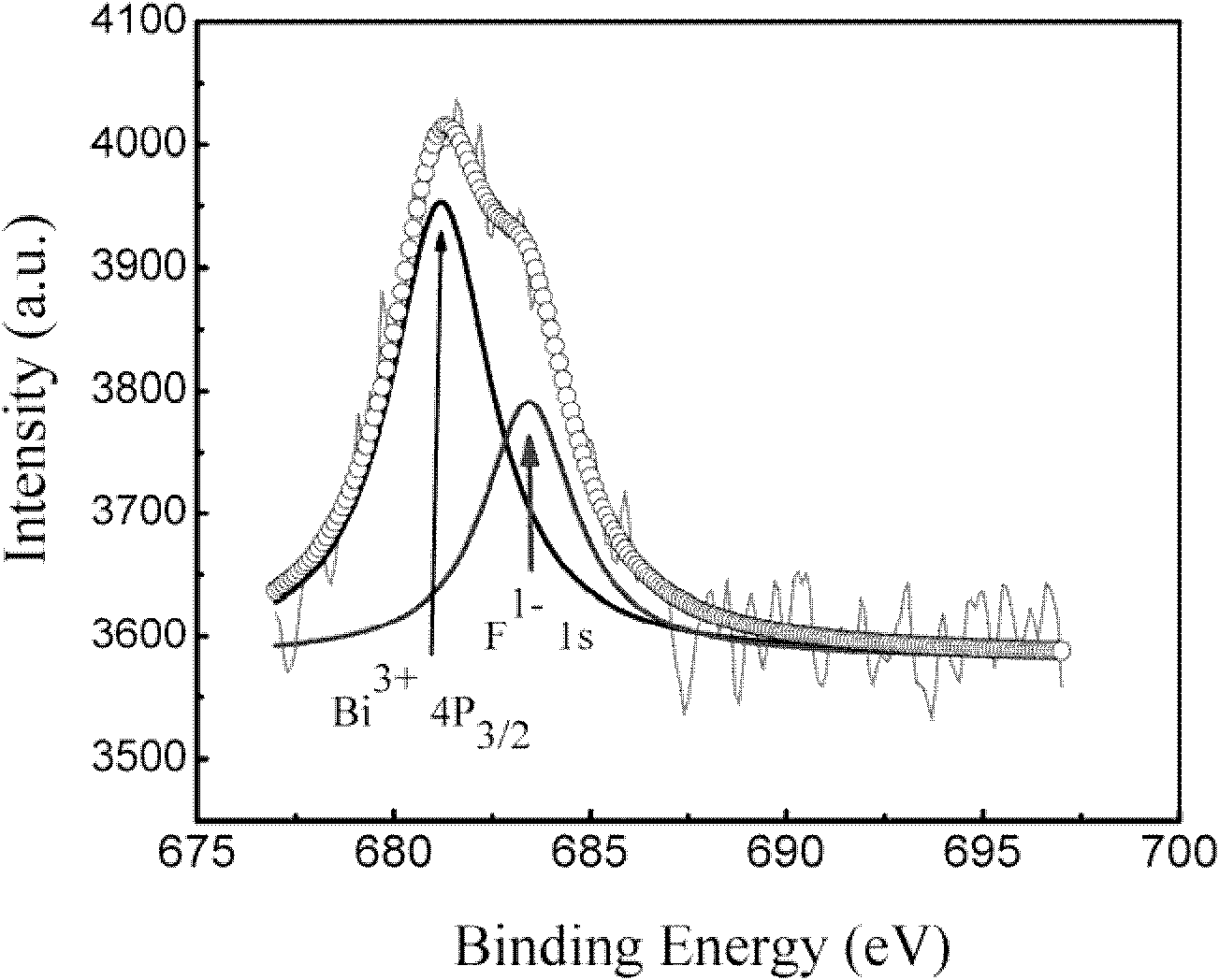
[0030] After the sample preparation was completed, in order to detect the quality of the sample, we performed X-ray diffraction (X-ray diffraction, XRD) analysis, and its XRD pattern is as attached figure 2 Shown in x=0.2. The XRD results show that the phase formation degree of the sample is very good. In order to detect whether F effectively enters the bismuth ferrite lattice, its performance is significantly enhanced, and its hysteresis loop at room temperature is measured, as shown in the attached Figure 5 Shown in X=0.2. The results show that: after F enters the bismuth ferrite lattice effectively, it exhibits obvious ferromagnetism.
Embodiment 3
[0031] Embodiment 3: the content of synthesizing F is the sample of 0.15
[0032] Attached figure 1 The preparation process shown, the synthesis of BiFeO with a F content of 0.15 2.85 f 0.15 sample. Its synthesis steps are the same as those in Example 1.
[0033] After the sample preparation was completed, in order to detect the quality of the sample, we performed X-ray diffraction (X-ray diffraction, XRD) analysis, and its XRD pattern is as attached figure 2 Shown in x=0.15. The XRD results show that the phase formation degree of the sample is very good. In order to detect whether F effectively enters the bismuth ferrite lattice, its performance is significantly enhanced, and its hysteresis loop at room temperature is measured, as shown in the attached Figure 5 Shown in X=0.15. The results show that: after F enters the bismuth ferrite lattice effectively, it exhibits obvious ferromagnetism.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com