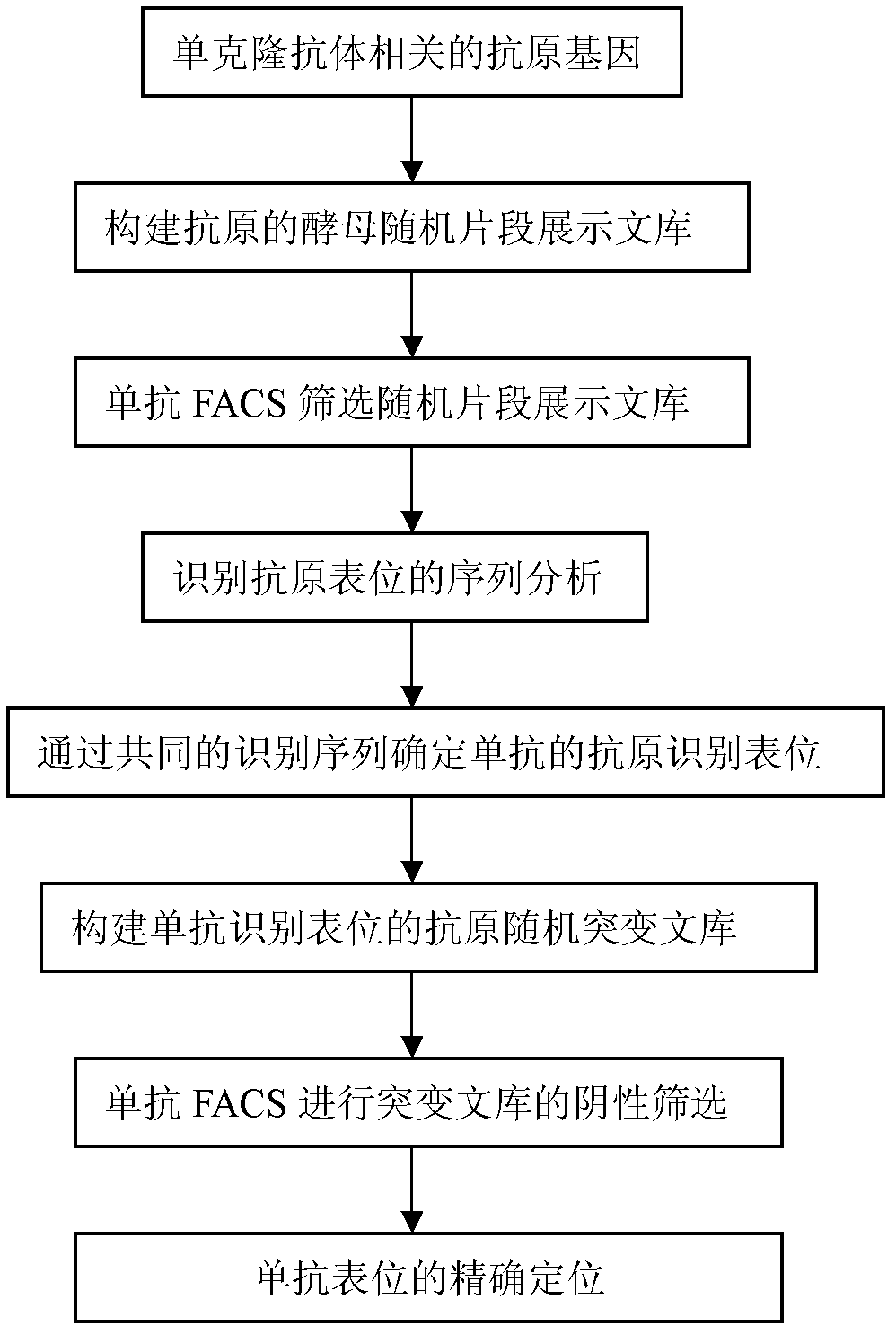
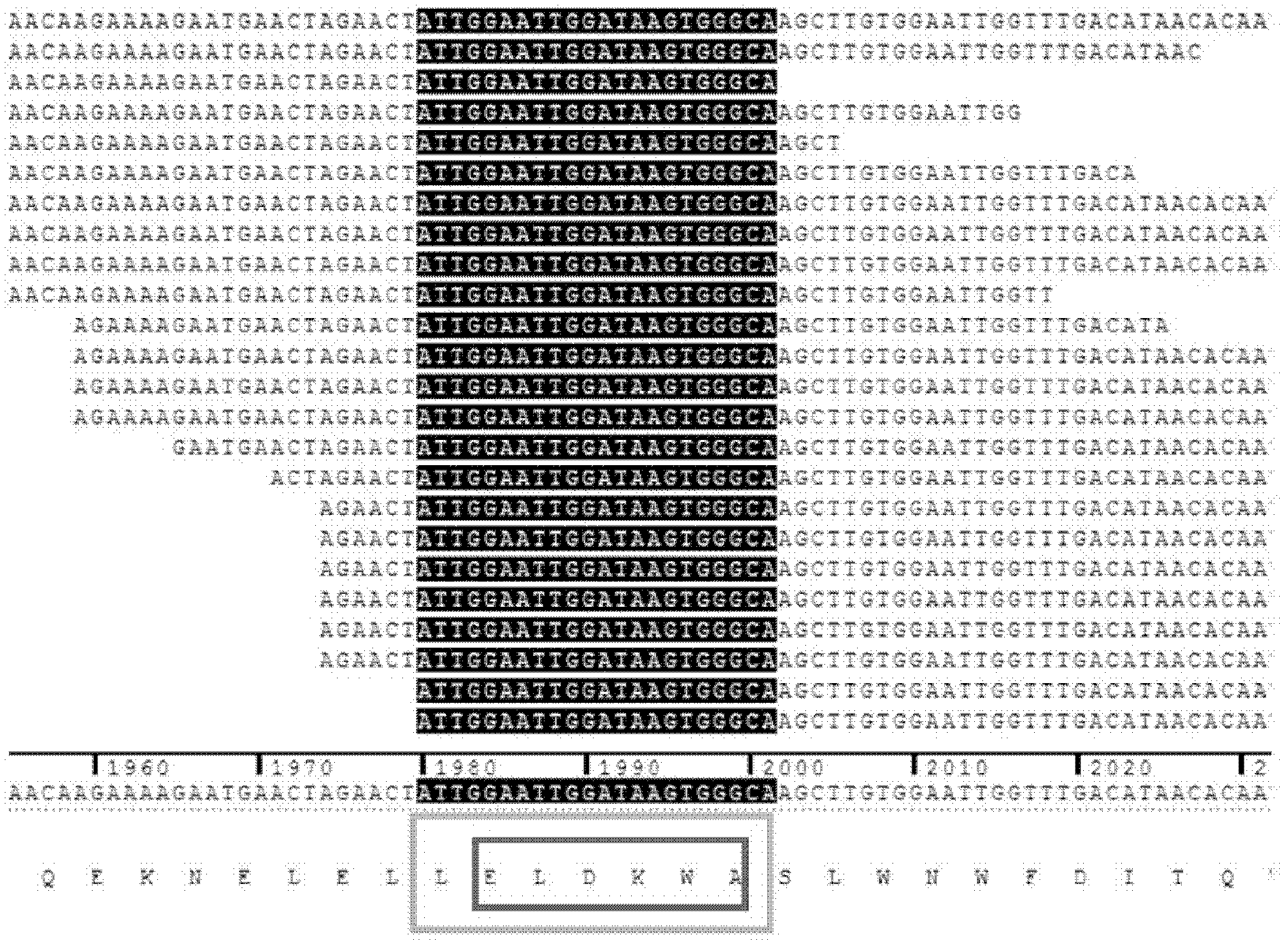Method for analyzing epitope of monoclonal antibody by using yeast surface display system and application of method in vaccine development
An antigenic epitope, yeast technology, applied in the preparation methods of peptides, chemical instruments and methods, and the introduction of foreign genetic material using vectors, can solve the problems of easy airborne phage transmission, small phage individuals, cross-contamination, etc. The effect of accuracy and screening throughput, ease of operation, and few interference factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1, the construction of pCTCON2-T plasmid (recombinant yeast display vector)
[0069] In order to facilitate library construction, the pCTCON2 plasmid needs to be transformed into a T vector, and the steps are as follows:
[0070] 1. Synthesize the DNA (double strand) shown in sequence 5 of the sequence listing.
[0071] 5'-AGTC GCTAGC GGATCC GGGT-3' (SEQ ID NO: 5).
[0072] The Nhe I recognition sequence is underlined on the left, the BamH I recognition sequence is marked on the right underline, and the two Xcm I recognition sequences are marked in bold.
[0073] 2. Digest the DNA synthesized in step 1 with restriction endonucleases Nhe I and BamH I, and recover the digested product.
[0074] 3. Digest the pCTCON2 plasmid with restriction endonucleases Nhe I and BamH I, and recover the vector backbone.
[0075] 4. Ligate the digested product of step 2 with the vector backbone of step 3 to obtain the pCTCON2-T plasmid (the backbone vector is a pCTCON2...
Embodiment 2
[0076] Embodiment 2, the preparation of AVFluIgG03 monoclonal antibody
[0077] 1. Synthesize the light chain variable region gene shown in sequence 6 in the sequence listing, and clone it into the Pac-L-Fc vector (PROGEN PR3003, Germany) between the Xba I and Sac I restriction sites to obtain recombinant plasmid A.
[0078] 2. Synthesize the heavy chain variable region gene shown in sequence 7 in the sequence listing, and clone it into the space between the Xho I and SpeI restriction sites of the recombinant plasmid A to obtain the recombinant plasmid B.
[0079]3. Using the BaculoGold co-transfection kit from Pharmogen, USA, the recombinant plasmid B was introduced into Sf9 cells, and the culture supernatant was collected to obtain AVFluIgG03 monoclonal antibody.
Embodiment 3
[0080] Embodiment 3, application method of the present invention analyzes the epitope of gp160 protein
[0081] 1. Construction of DNA library of gp160 protein (random cutting fragment library)
[0082] To construct the DNA library of the gp160 protein, first the coding gene of the gp160 protein is randomly cut by DNase I to obtain small fragments of about 50-100bp, and then a random fragment DNA library with a specific length distribution is obtained by random recombination (Lin, Z., S. Li and Y. Chen, Identification of Viral Peptide Fragments for Vaccine Development. Viral Applications of Green Fluorescent Protein: Methods and Protocols, 2009: p.261-274.; Chen, Y., et al., Random dissection to select for protein split sites and its application in protein fragment complementation. PROTEIN SCIENCE, 2009.18(2): p.399-409.). The above method is very similar to the program of DNA shuffling (Lorimer, I.and I.Pastan, Random recombination of antibody single-chain fv sequences after...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com