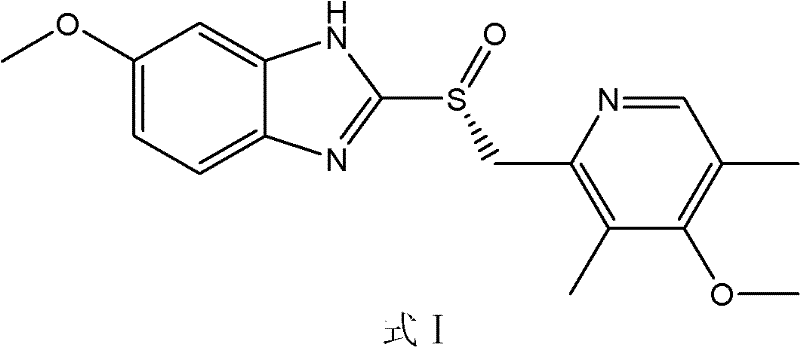
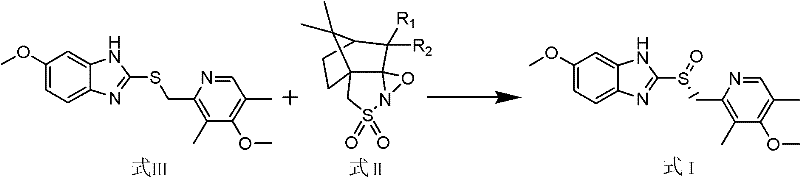
Method for preparing esomeprazole and salts thereof
A technology of esomeprazole and omeprazole sulfide, applied in the direction of organic chemistry and the like, can solve the problems of serious environmental pollution and high cost, and achieve the effects of high stereoselectivity, low production cost and stable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
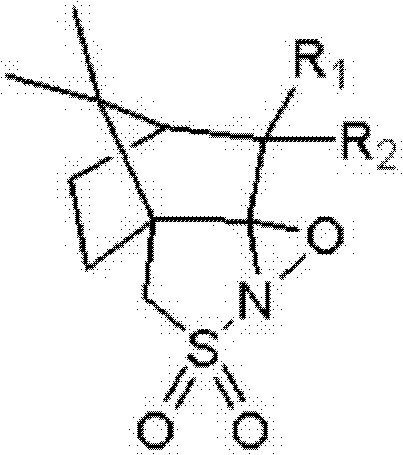
[0045] Dissolve 5g of omeprazole sulfide in 50mL of isopropanol, add 1.54g of triethylamine, stir and react for 30 minutes at room temperature, then add 1.74g of (1R)-(- )-camphorsulfonazine, stirring and reacting for 20 hours, TLC (developing solvent: acetone: petroleum ether=1: 2, color development: ultraviolet) detected that the reaction was complete. Suction filtration, wash the filter cake with isopropanol, add about 60mL of water after the filtrate is concentrated, adjust its pH to 7-9 with hydrochloric acid solution, extract 3 times with ethyl acetate, take the organic phase, concentrate and dry to obtain foamy solid Esso Meprazole 3.96g, yield: 75.5%. HPLC assay ee: 99.27%.
Embodiment 2
[0047] Dissolve 5g of omeprazole sulfide in 80mL of toluene, add 4.62g of DBU, stir and react for 60 minutes at room temperature, then add 3.48g of (1R)-(-)-methyl Camphorsulfonazine was stirred and reacted for 6 hours, and the reaction was detected to be complete by TLC (developer: acetone:petroleum ether=1:2, color development: ultraviolet). Filtrate with suction, wash the filter cake with toluene, add about 40mL of water after the filtrate is concentrated, adjust its pH to 7-9 with acetic acid solution, extract 4 times with dichloromethane, take the organic phase, concentrate, and dry to obtain esomemera as a foamy solid Azole 4.08g, yield: 77.8%. HPLC assay ee: 99.24%.
Embodiment 3
[0049] Dissolve 5g of omeprazole sulfide in 100mL of ethanol, add 3.04g of sodium hydroxide, stir and react for 90 minutes at room temperature, then add 6.96g of (1R)-(-)-camphorsulfonate at 75°C to 80°C Dumbazine was stirred and reacted for 2 hours, and the reaction was detected to be complete by TLC (developer: acetone:petroleum ether=1:2, color development: ultraviolet). Suction filtration, wash the filter cake with ethanol, add about 80mL water after the filtrate is concentrated, adjust its pH to 7-9 with propionic acid solution, extract 3 times with ethyl acetate:toluene (1:1), take the organic phase to concentrate, dry, 4.16 g of foamy solid esomeprazole was obtained, yield: 79.3%. HPLC assay ee: 99.36%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com