Method for preparing isobutylaldehyde by performing selective hydrogenation on methylacrolein
A technology for the selective hydrogenation of methacrolein, applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds, to achieve good stability, mild reaction conditions, and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
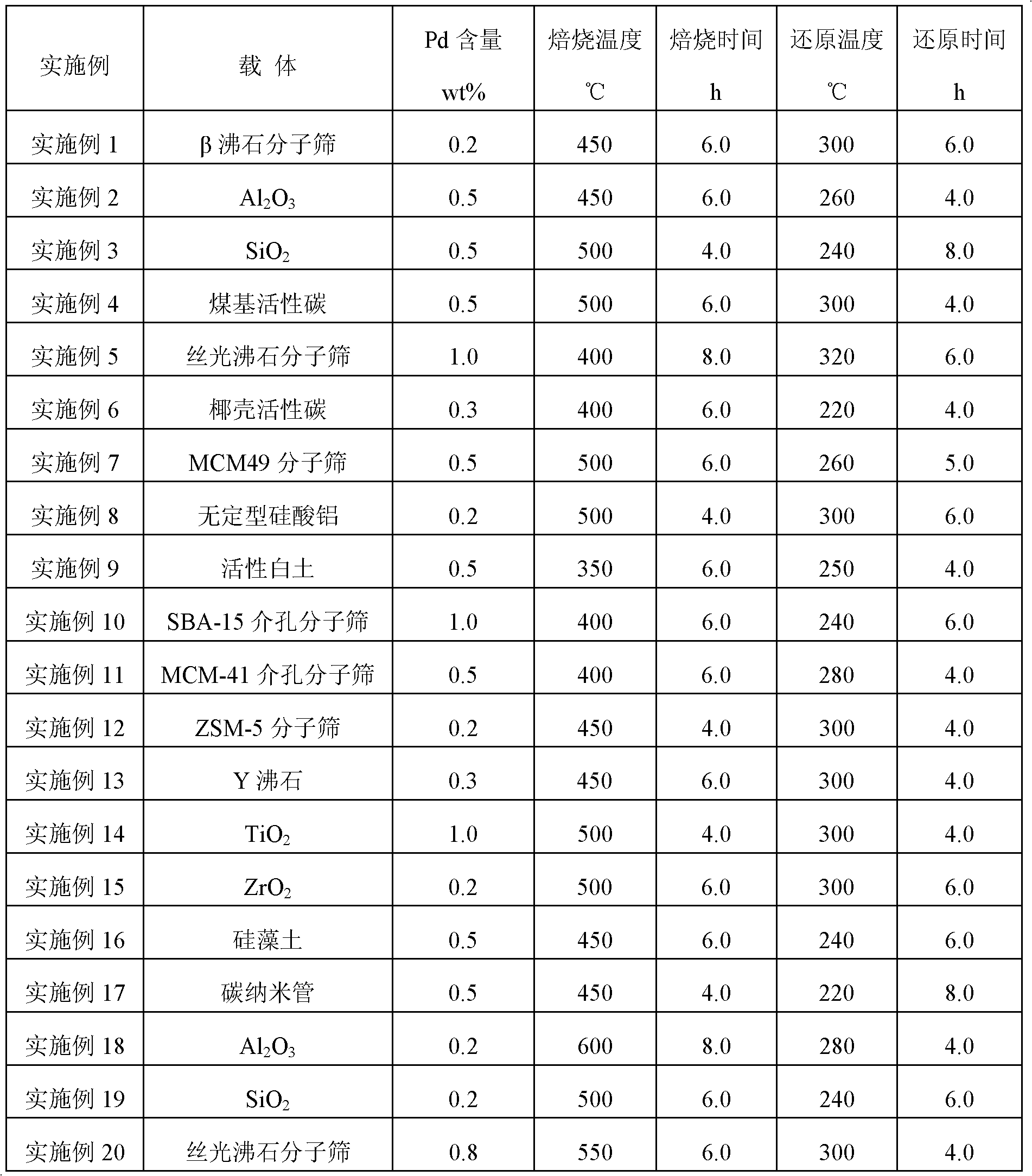
[0018] One, the preparation of catalyst:
[0019] Prepare a certain concentration of Pd-containing soluble brine solution, add it to the corresponding carrier for impregnation according to the metering ratio, and prepare the catalyst precursor, then dry it at 110 ° C for 12 hours, after high-temperature roasting, cool it down to room temperature, and put it into the reactor. Use hydrogen or hydrogen-nitrogen mixed gas at a pressure of 1.0MPa and a hydrogen space velocity of 1000h -1 Reduction treatment under the same conditions can obtain the supported Pd catalyst of the present invention.
Embodiment 1~20
[0021] The supported Pd catalyst prepared above is adjusted to the reaction conditions, and the feed is reacted. The catalyst carrier, Pd content, preparation conditions, target product reaction conditions, and reaction results are shown in Table 1, Table 2, and Table 3, respectively.
[0022] Table 1
[0023]
[0024] 2. Evaluation of the reactivity of the catalyst:
[0025] 1. Catalyst tank reaction activity evaluation:
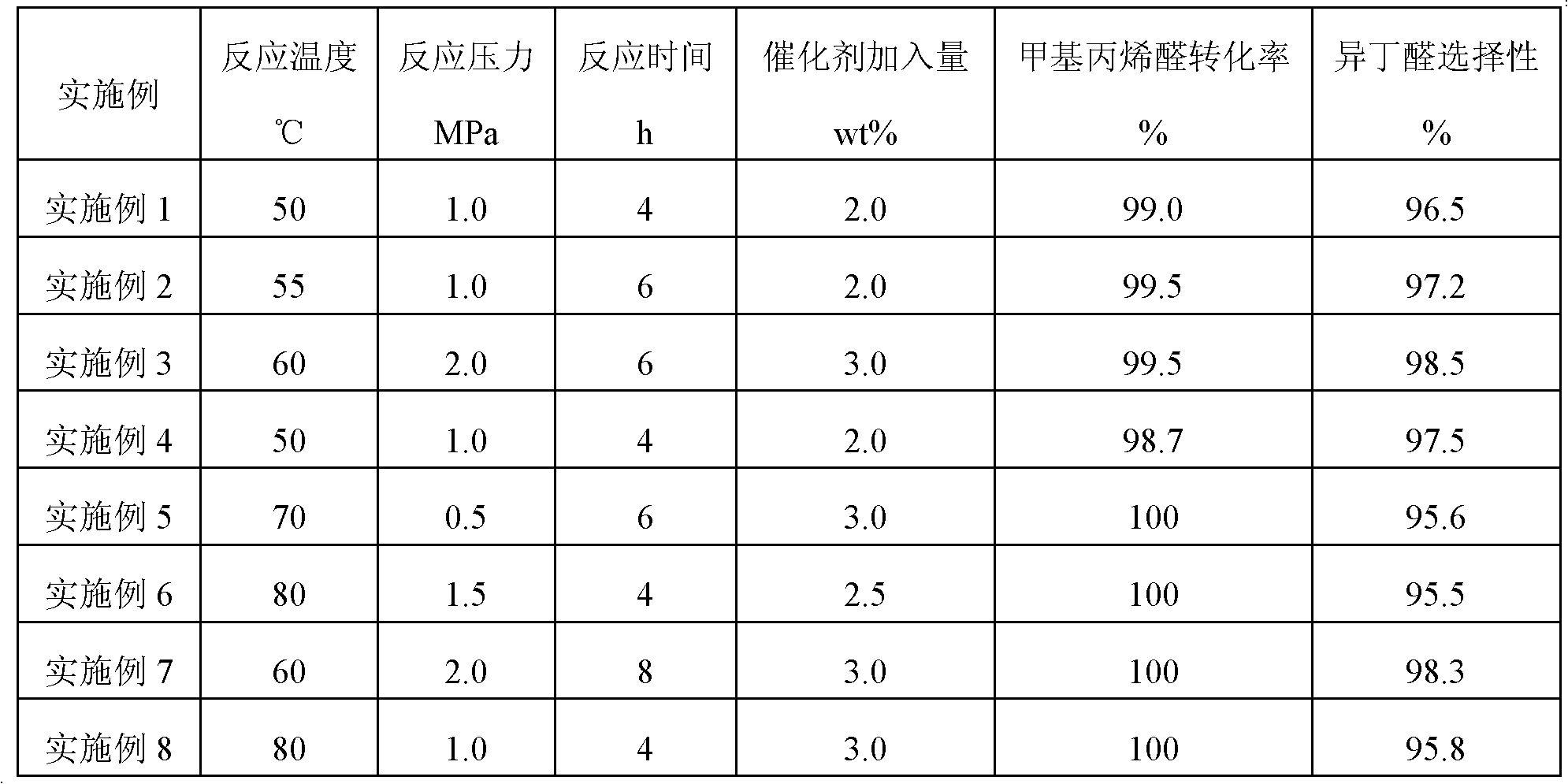
[0026] In a 500 milliliter stainless steel high-pressure stirred reactor, add 300 grams of methacrolein and a certain amount (calculated based on the reaction raw materials) of one of the catalysts in the above-mentioned examples 1 to 8, and heat up to Reaction temperature, control reaction pressure 0.5~10MPa, reaction temperature 50~100 ℃, stirring speed 500~2000 rpm. The specific reaction conditions and reaction results in Examples 1-8 are shown in Table 2.
[0027] Table 2
[0028]
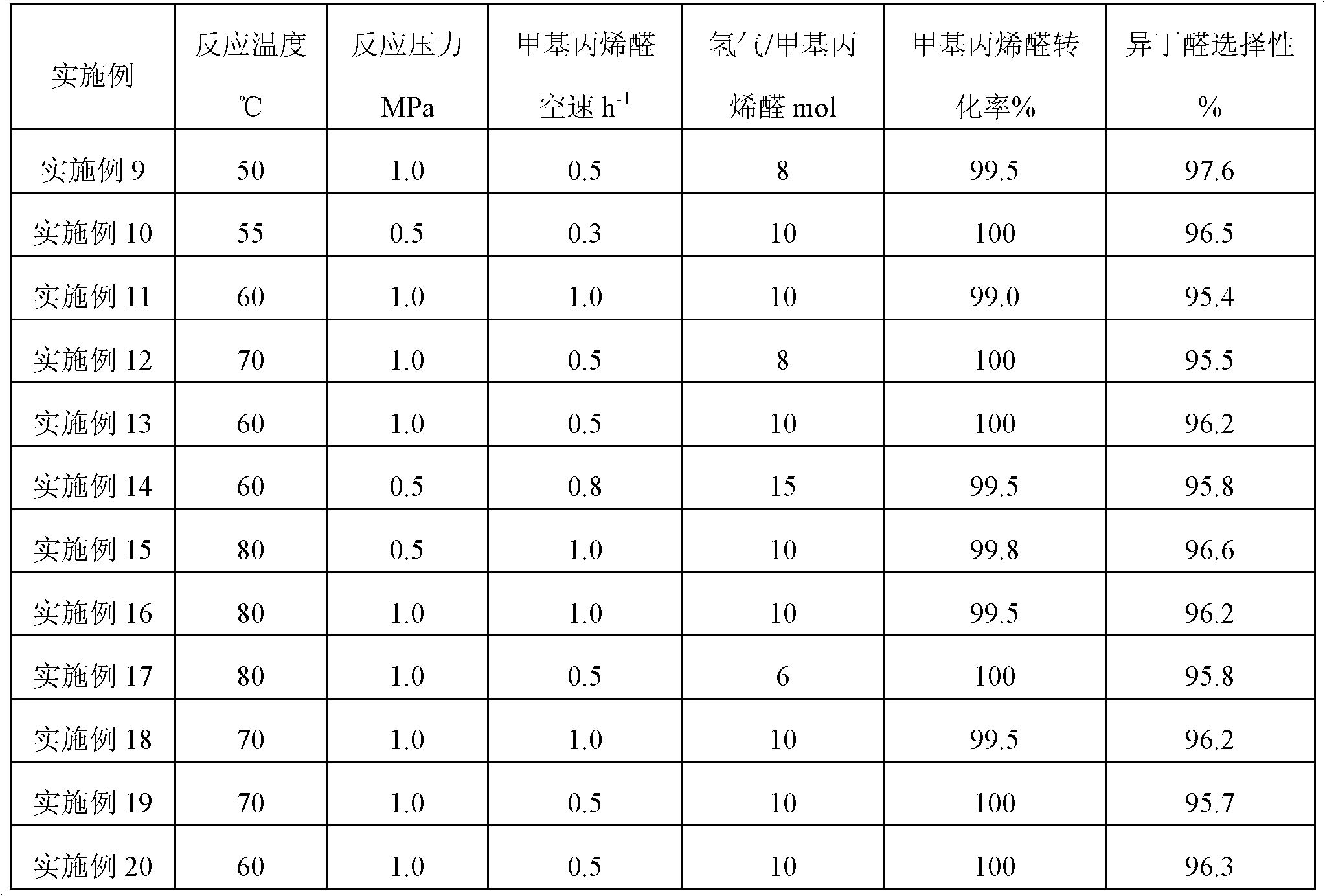
[0029] 2. Evaluation of the fixed bed tubular reaction activ...
Embodiment 21~22
[0035] 100 grams of the catalysts in Examples 2 and 5 were respectively loaded into a fixed-bed tubular reactor, and reduced according to the corresponding conditions in Table 1. The methacrolein raw material and hydrogen were mixed and preheated and then entered into the reactor for reaction to investigate the stability of the catalyst. The reaction conditions were the same as in Examples 2 and 5 respectively. The reaction results are shown in Table 4.
[0036] Table 4
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com