Immunochromatography detection reagent strip for combined detection of toxoplasmagondii IgG antibodies and total antibodies, and preparation method thereof
A joint detection and Toxoplasma gondii technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of high false positive, low value, poor sensitivity of IgM antibody, etc., and achieve simple and fast detection, wide application and strong specificity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
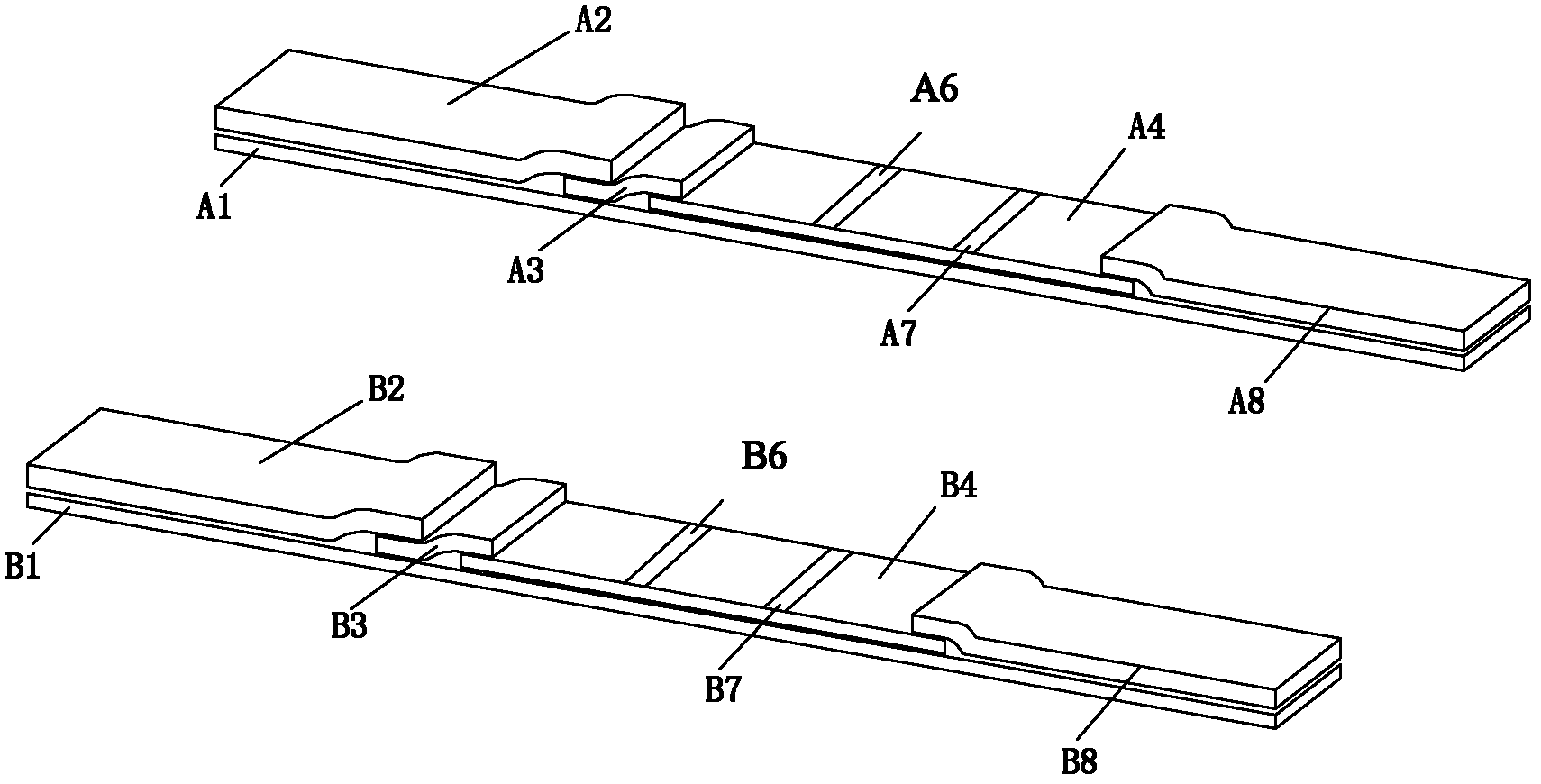
[0041] The preparation method of the combined detection reagent strip of the toxoplasma IgG antibody and the total antibody comprises the following steps:
[0042] 1) Preparation of Toxoplasma gondii recombinant antigens SAG1 (P30), SAG2 (P22), ROP2 and GRA7
[0043] Using gene cloning technology, PCR amplifies DNA encoding Toxoplasma gondii antigen, inserts it into Escherichia coli for expression, and obtains Toxoplasma gondii recombinant antigens SAG1 (P30), SAG2 (P22), ROP2 and GRA7.
[0044] 2) Spotting on nitrocellulose membrane
[0045] Coat anti-human IgG specific fragment gamma chain monoclonal antibody on the detection line of Toxoplasma IgG antibody, coat anti-human Ig monoclonal antibody at the total antibody detection line, dry, the concentration of described anti-human Ig monoclonal antibody The concentration of anti-human IgG specific fragment γ chain monoclonal antibody can be 1-4 mg / mL, the IgG antibody of goat anti-toxoplasma antigen SAG1 (P30), SAG2 (P22), R...
Embodiment 1
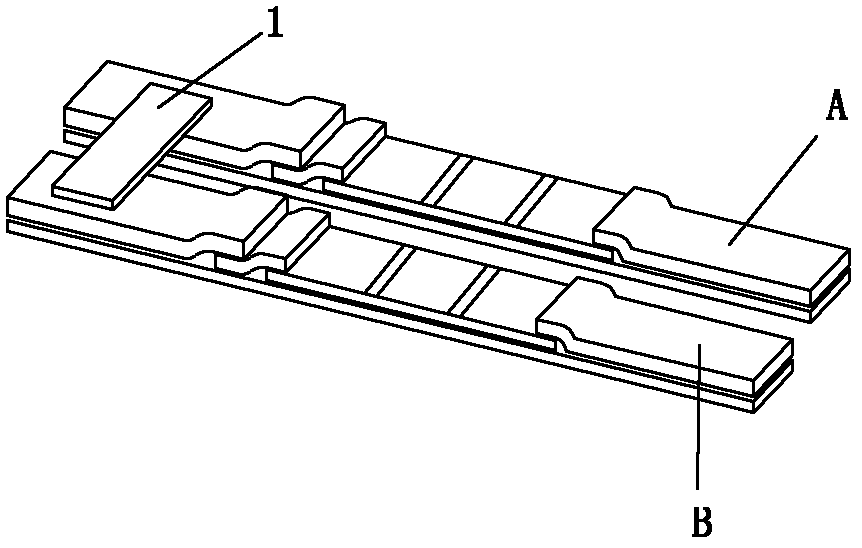
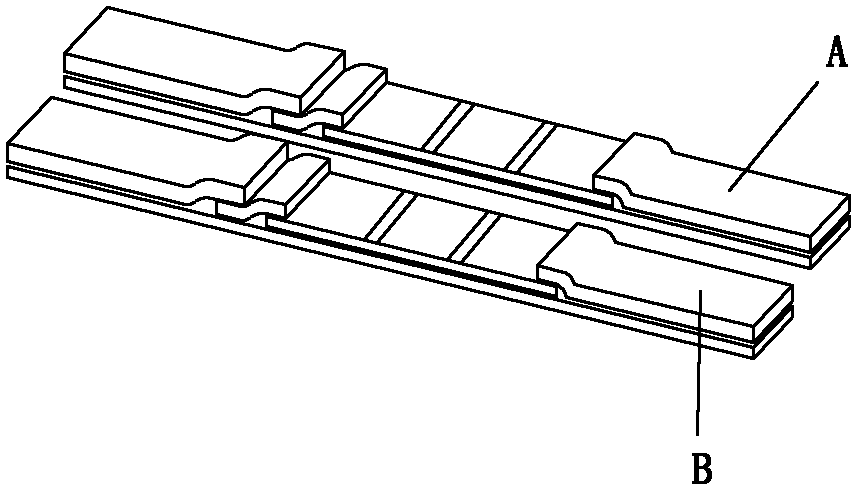
[0071] The first reagent strip A is coated with anti-human IgG specific fragment γ chain monoclonal antibody on the nitrocellulose membrane (NC membrane) IgG detection line, and the second reagent strip B is coated with anti-human Ig monoclonal antibody on the total antibody detection line Antibodies, coated with goat anti-toxoplasma antigens SAG1 (P30), SAG2 (P22), ROP2 and GRA7 antibodies at the control line C, dried at room temperature, sealed and stored at room temperature for future use. Among them, the concentration of anti-human IgG specific fragment γ chain monoclonal antibody and anti-human Ig monoclonal antibody is 1 mg / mL, and goat anti-toxoplasma antigen (SAG1 (P30), SAG2 (P22), ROP2 and GRA7) IgG antibody is obtained by Goat anti-toxoplasma antigen (SAG1(P30), SAG2(P22), ROP2 and GRA7) IgG antibody consists of anti-SAG1(P30)-IgG antibody, anti-SAG2(P22)-IgG antibody, anti-ROP2-IgG antibody and anti-GRA7- IgG antibodies were mixed at a volume ratio of 1:1:1:1, and ...
Embodiment 2
[0075] Similar to Example 1, the difference is that the gold colloid pad and the total antibody detection line of Toxoplasma gondii are only composed of SAG1(P30), SAG2(P22), ROP2 and GRA7, and do not contain SAG1(P30), SAG2(P22), ROP2 and GRA7 . Result judgment is identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com