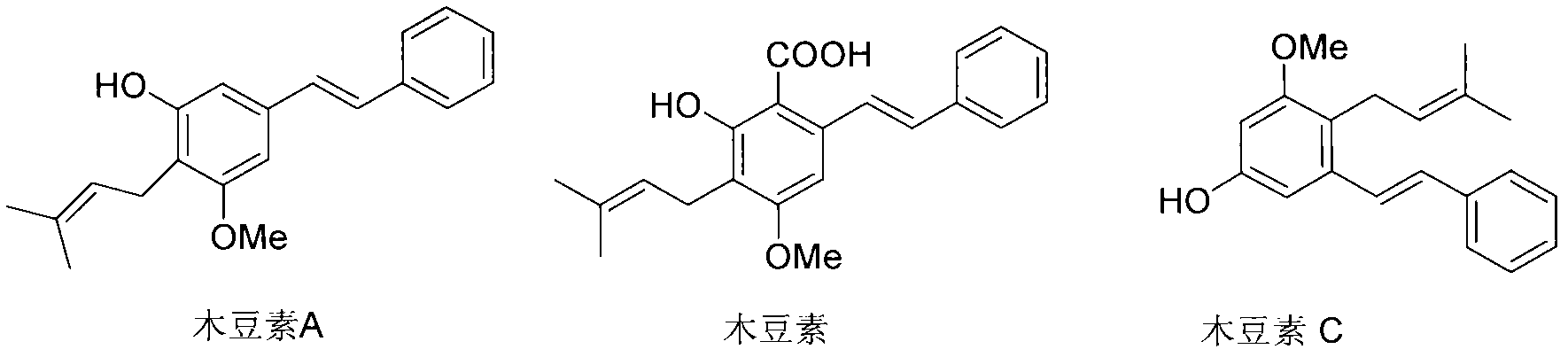
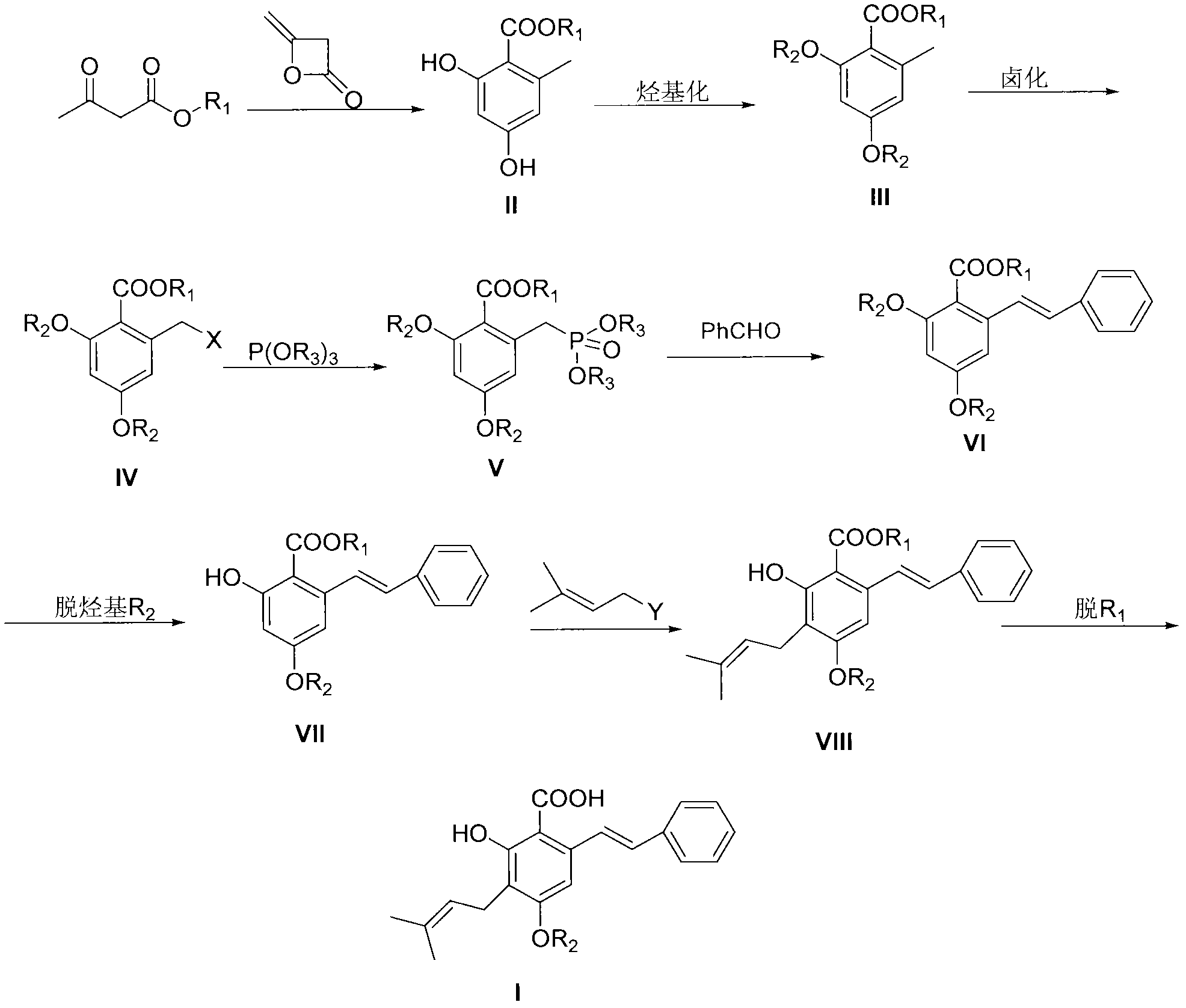
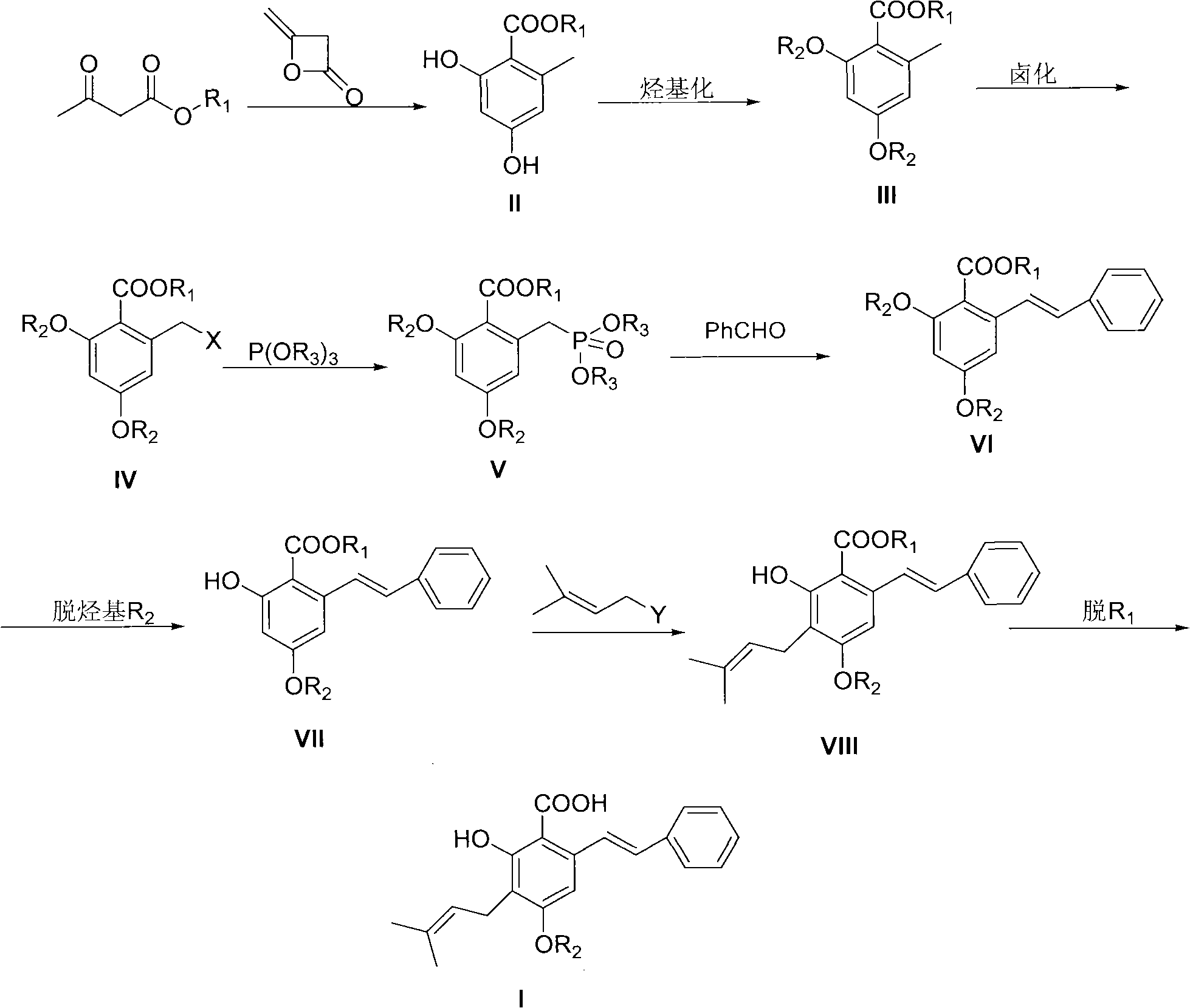
Preparation method of cajanin and substance with similar structure
A technology similar to kalinin, which is applied in the preparation of organic compounds, preparation of oxygen-containing compounds, chemical instruments and methods, etc., can solve the problems of no literature reports on the total synthesis of kalinin and its structural analogues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]2, the preparation of 4-dihydroxy-6-methylbenzoic acid methyl ester (II, R 1 for methyl)
[0043] Methyl acetoacetate (50g, 0.43mol) was dissolved in 300ml of ether, NaH (15.50g, 0.45mol, 70%) was added at room temperature, after the addition was complete, diketene (37g, 0.45mol) was added dropwise at this temperature in ether solution , After the addition, react at room temperature for 3-4h, when the reaction system turns into a yellow turbid liquid. Terminate the reaction, add the reaction liquid to 500ml of ice-water mixture, separate the ether layer, extract the water layer twice with 50ml of ether, combine the ether layers, wash with saturated brine, and dry over anhydrous magnesium sulfate. Filtration, rotary evaporation to remove diethyl ether, the residue was passed through the column with petroleum ether: ethyl acetate = 8:1 to obtain 35 g of white solid which was the target product (yield 45%).
[0044] 1 H-NMR (400M, DMSO-d 6 ): δ(ppm) 2.26(s, 3H), 3.78(s,...
Embodiment 2
[0046] 2, the preparation of 4-dimethoxy-6-methylbenzoic acid methyl ester (III, R 1 , R 2 both methyl)
[0047] Compound II (12g, 0.066mol) was dissolved in 50ml of acetone, potassium carbonate (27.3g, 0.198mol) and methyl iodide (28g, 0.198mol) were added, heated to reflux for 3 hours, and the reaction was stopped. Extract with ethyl ester (3*50ml), combine the organic layers, and wash the organic layer successively with 10% sodium hydroxide solution, 10% hydrochloric acid, and saturated brine. The organic layer was dried over anhydrous magnesium sulfate. After filtration, the solvent was removed by rotary evaporation to obtain a colorless oil, which was recrystallized from petroleum ether / ethyl acetate to obtain 13.5 g of colorless crystals (97% yield).
[0048] 1 H-NMR (400M, CDCl 3 ): δ (ppm) 2.28 (s, 3H), 3.80 (s, 6H), 3.88 (s, 3H), 6.31 (s, 2H).
Embodiment 3
[0050] Methyl 2,4-dimethoxy-6-bromomethylbenzoate (IV, R 1 , R 2 Both are methyl, X is bromine)
[0051] Compound III (10g, 0.0476mol) was dissolved in 50ml of carbon tetrachloride, under nitrogen protection, heated to reflux, and a mixture of NBS (8.5g, 0.0476mol) and BPO (0.11g, 0.476mmol) was added in batches, after the addition was complete, reflux Reaction 1h. The reaction was stopped, cooled, filtered, and the filtrate was spin-dried to obtain a light yellow solid, which was recrystallized from absolute ethanol to obtain 11.3 g of a white solid (yield 82%).
[0052] 1 H-NMR (400M, CDCl 3 ): δ(ppm) 3.85(s, 3H), 3.93(s, 3H), 3.96(s, 3H), 4.66(s, 2H), 6.47(s, 1H), 6.74(s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com