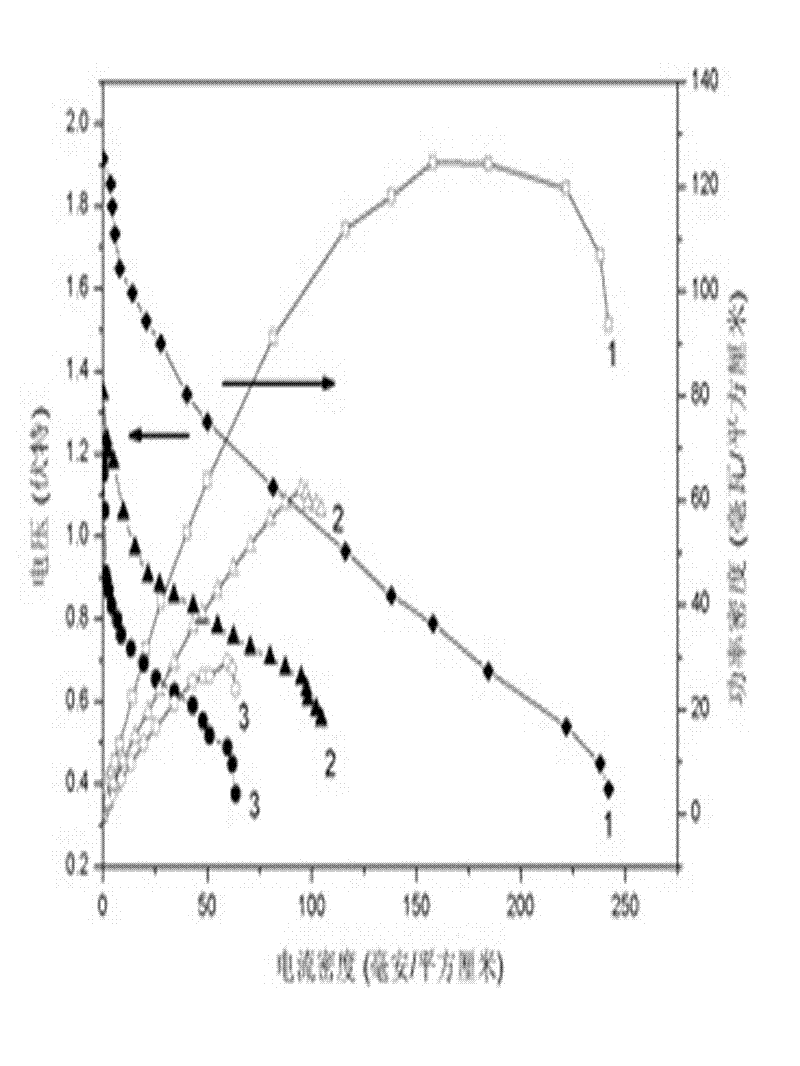
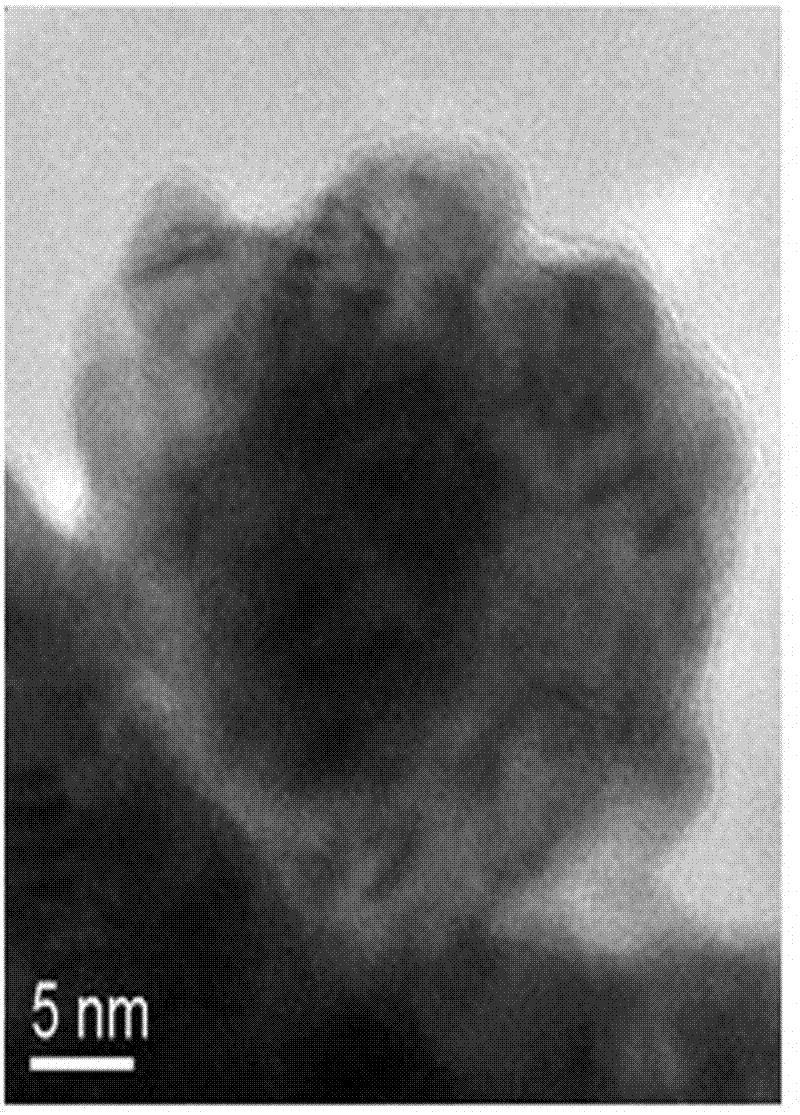
Core-shell structural anode catalyst for direct borohydride fuel cells and preparation method thereof
A core-shell structure, borohydride technology, used in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, battery electrodes, etc. It can improve the utilization rate, high activity and less hydrogen evolution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Preparation of Cu nanoparticles: Cu(NO 3 )·3H 2 O and polyvinylpyrrolidone (PVP) were sequentially added to 20 ml of ethylene glycol, so that the concentration of Cu atoms in ethylene glycol was 20 mmol / L, and polyvinylpyrrolidone (PVP) in ethylene glycol The concentration is 3 g / L. Stir and pass nitrogen gas for 25 minutes to make it fully mixed, then heat to 60°C, continue to pass nitrogen gas and stir continuously, and the dropwise concentration is 0.3 mol L -1 Hydrazine hydrate solution, the molar amount of hydrazine hydrate added is twice that of Cu element, and the dropping rate is 40 drops·min -1 , reacted for 40 min to prepare Cu nano catalyst sol, then suction filtered and washed with distilled water to obtain Cu nanoparticles;
[0033] (2) Re-dissolve the Cu nanoparticles in the above (1) in 20 ml of ethylene glycol, add polyvinylpyrrolidone (PVP) under stirring to make the concentration 3 g / L, blow in nitrogen and stir For 30 minutes, add a tetrahydro...
Embodiment 2
[0036] (1) Preparation of Ni nanoparticles: NiCl 2 ·6H 2 O and tetraoctylammonium bromide were added to 20ml of water in turn, so that the concentration of Ni atoms in the water was 10 mmol / L, and the concentration of tetraoctylammonium bromide in water was 1.5 g / L. Stir and pass nitrogen gas for 20 minutes to make it fully mixed, then heat to 50°C, continue to pass nitrogen gas and stir constantly, the dropwise concentration is 0.3 mol L -1 Lithium triethyl borohydride solution, adding lithium triethyl borohydride molar weight is 1.5 times that of Ni element, and the dropping speed is 40 drops min -1 , and reacted for 40 min to prepare Ni nano catalyst sol, then suction filter and wash with distilled water to obtain Ni nanoparticles;
[0037] (2) Re-dissolve the M nanoparticles in the above (1) in 20 ml of water, add tetraoctyl ammonium bromide under stirring, the concentration of tetraoctyl ammonium bromide is 1.5g / L, blow in nitrogen and stir for 30 Minutes, then add a t...
Embodiment 3
[0040] (1) Preparation of Pt nanoparticles: H 2 PtCl 6 ·6H 2 O and polyethylene glycol were sequentially added to 20 ml of tetrahydrofuran, so that the concentration of Pt atoms in tetrahydrofuran was 30 mmol / L, and the concentration of polyethylene glycol in tetrahydrofuran was 5 g / L. Stir and pass nitrogen gas for 30 minutes to make it fully mixed, then heat to 80°C, continue to pass nitrogen gas and stir constantly, the dropwise concentration is 0.3 mol L -1 Sodium borohydride solution, the molar weight of sodium borohydride added is 2.5 times that of Pt element, and the dropping rate is 40 drops·min -1 , reacted for 60 min, prepared Pt nano catalyst sol, then suction filtered, and washed with distilled water to obtain Pt nanoparticles;
[0041] (2) Redissolve the Pt nanoparticles in the above (1) in 20ml tetrahydrofuran, add polyethylene glycol under stirring, the concentration of polyethylene glycol is 5g / L, blow in nitrogen and stir for 30 minutes, and then , the mol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Maximum power density | aaaaa | aaaaa |
| Maximum power density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com