High capacity cathode material of lithium ion battery and preparation method thereof
A technology for lithium-ion batteries and positive electrode materials, which is applied in the direction of battery electrodes, circuits, electrical components, etc., can solve problems that have not been seen, and achieve the effects of shortened migration paths, strong adsorption capacity, and moderate hardness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
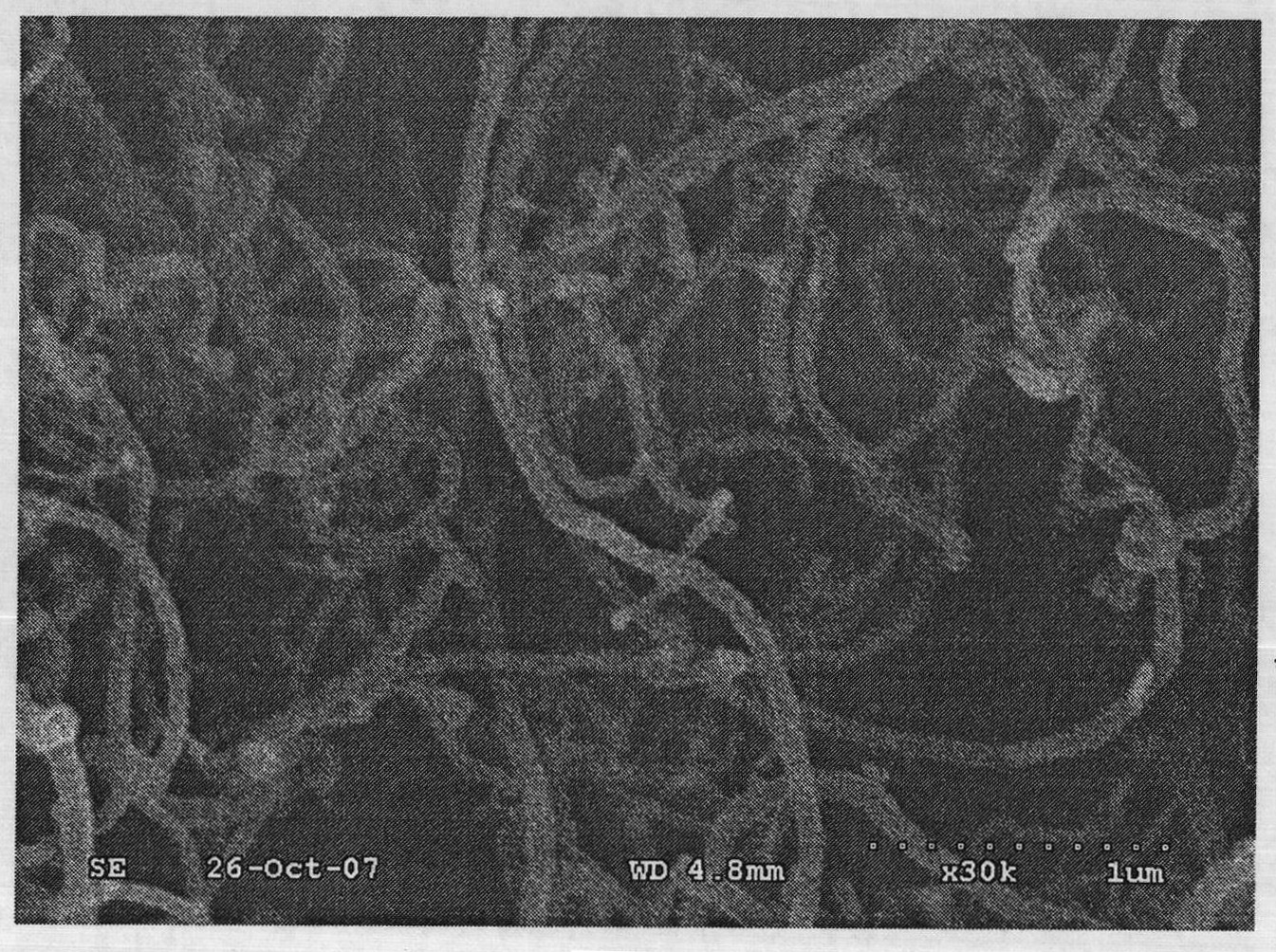
[0029] Example 1: Dissolve 5.1010 g of lithium acetate, 1.2941 g of nickel acetate, 5.1959 g of manganese acetate, and 1.2953 g of cobalt acetate in deionized water; add 1.5 g of cane charcoal and stir at 900 rpm for 1 hour. An aqueous solution containing 33 g of citric acid was added dropwise, and the pH value of the mixed system was adjusted to 10 with ammonia water, the reaction temperature was maintained at 70° C., and the stirring speed was 1200 rpm until a gel was formed. After the gel is dried, it is pre-calcined at 450°C for 4 hours, cooled and ground, calcined at 800°C for 8 hours, cooled and ground, and passed through a 200-mesh sieve to produce 0.5Li 2 MnO 3 0.5Li[Ni 1 / 3 mn 1 / 3 co 1 / 3 ]O 2 Material.
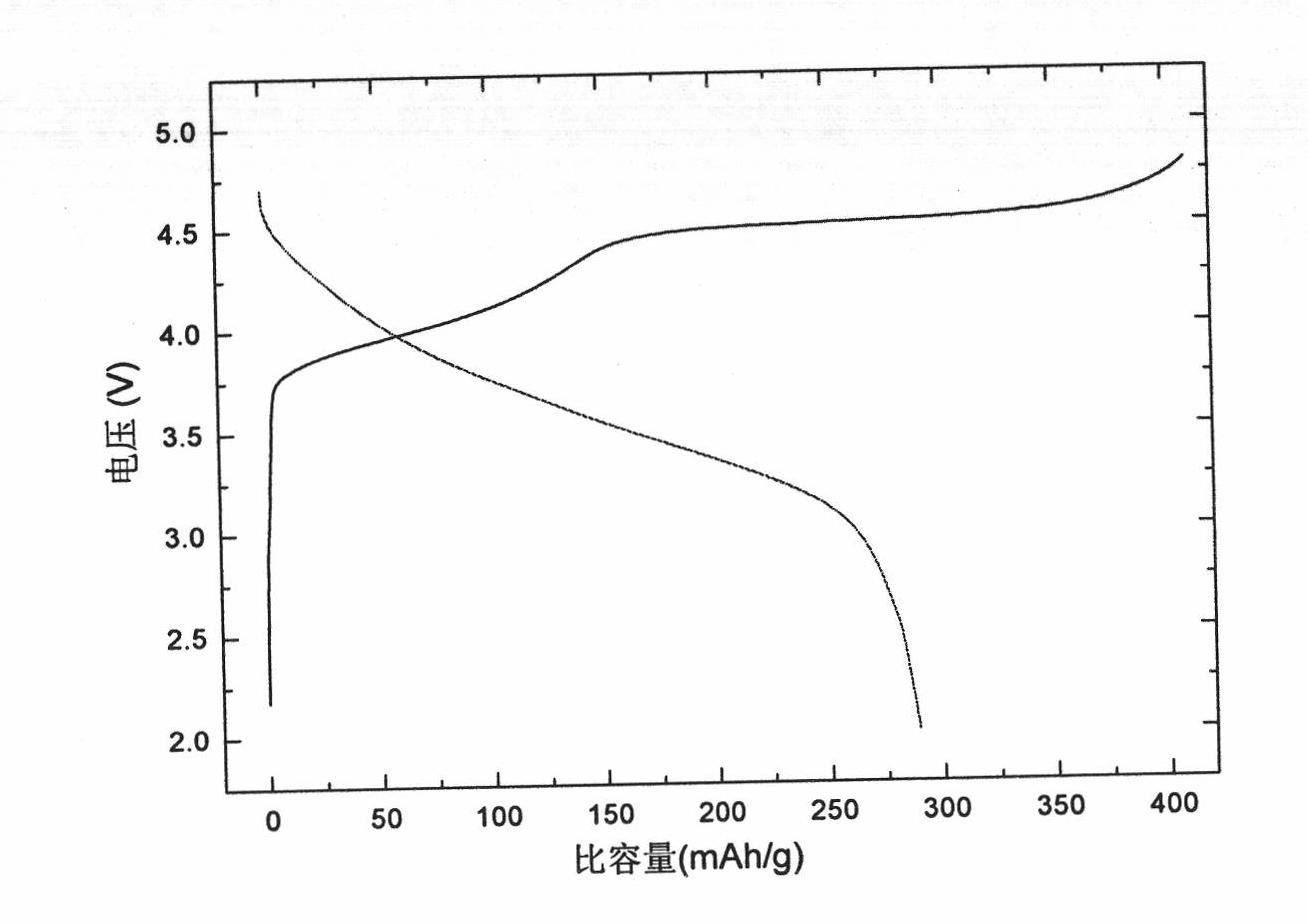
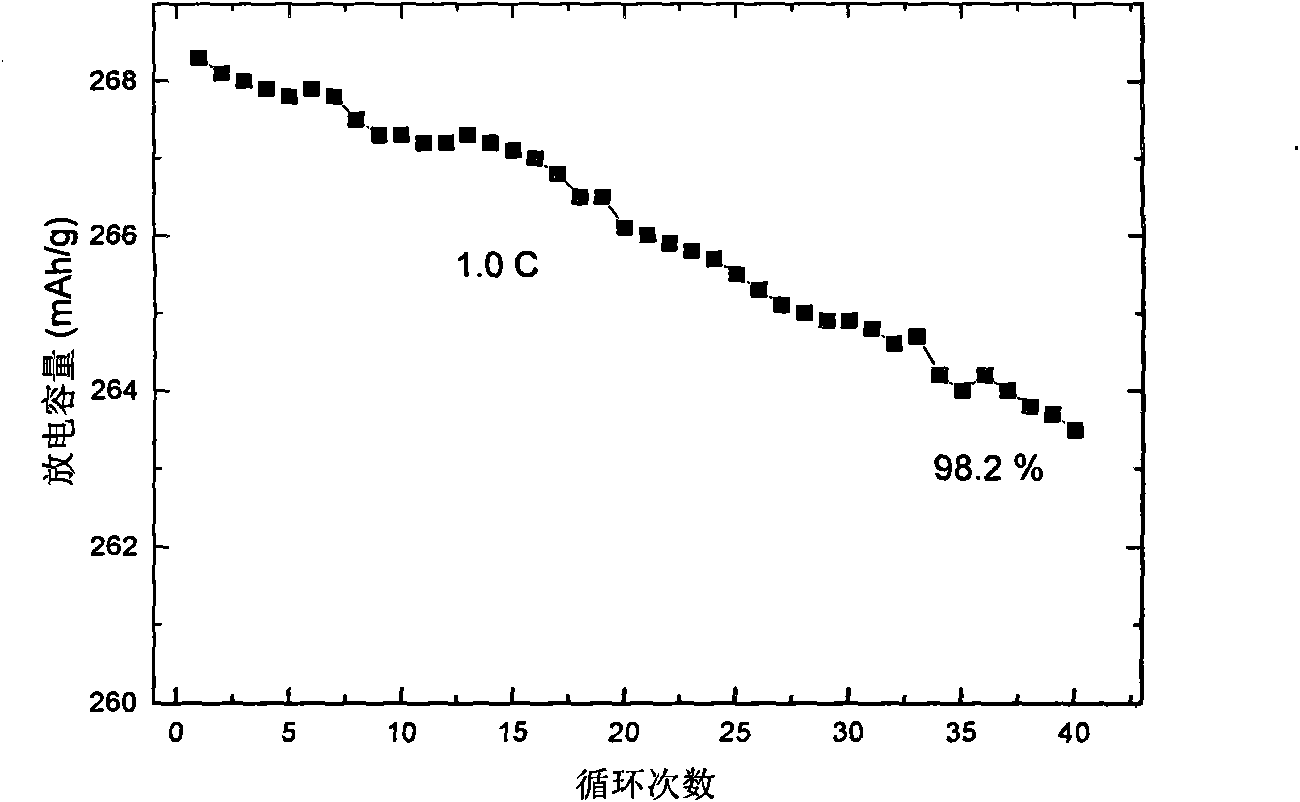
[0030] With the 0.5Li that embodiment 1 makes 2 MnO 3 0.5Li[Ni 1 / 3 mn 1 / 3 co 1 / 3 ]O 2 The material is mixed with the conductive agent carbon black and the binder polyvinylidene fluoride at a ratio of 85:10:5, and an appropriate amount of N-methylpyrrolidone ...
Embodiment 2
[0031] Example 2: Dissolve 4.76g of lithium oxalate, 1.2183g of nickel oxalate, 3.5798g of manganese oxalate and 3.6596g of cobalt oxalate in deionized water; add 2.5g of bamboo charcoal and stir at 1000 rpm for 0.5 hours. Then, an aqueous solution containing 9.6 g of polyvinyl alcohol was added dropwise, and the pH value of the mixed system was adjusted to 9 with urea, the reaction temperature was kept at 80° C., and the stirring speed was 1000 rpm until a gel was formed. After the gel is dried, calcined at 550°C for 3 hours, cooled and ground, calcined at 950°C for 9 hours, passed through a 200-mesh sieve after cooling and ground, and produced 0.4Li 2 MnO 3 0.6Li[Ni 1 / 3 mn 1 / 3 co 1 / 3 ]O 2 Material.
[0032] With the 0.4Li that embodiment 2 makes 2 MnO 30.6Li[Ni 1 / 3 mn 1 / 3 co 1 / 3 ]O 2 The material is mixed with the conductive agent carbon black and the binder polyvinylidene fluoride at a ratio of 85:10:5, and an appropriate amount of N-methylpyrrolidone solvent is ...
Embodiment 3
[0033] Example 3: Dissolve 3.3940 g of lithium nitrate, 1.1930 g of nickel nitrate, 6.4768 g of manganese nitrate, and 1.1940 g of cobalt nitrate in deionized water; add 1.6 g of loofah pulp and stir at 1500 rpm for 1.5 hours. An aqueous solution containing 24.62 g of sucrose was added dropwise, and the pH value of the mixed system was adjusted to 11 with ammonia water, the reaction temperature was kept at 90° C., and the stirring speed was 1500 rpm until a gel was formed. After the gel is dried, it is calcined at 650°C for 4 hours, cooled and ground, calcined at 850°C for 10 hours, cooled and ground, and passed through a 200-mesh sieve to produce 0.6Li 2 MnO 3 0.4Li[Ni 1 / 3 mn 1 / 3 co 1 / 3 ]O 2 Material.
[0034] With the 0.6Li that embodiment 3 makes 2 MnO 3 0.4Li[Ni 1 / 3 mn 1 / 3 co 1 / 3 ]0 2 The material is mixed with the conductive agent carbon black and the binder polyvinylidene fluoride at a ratio of 85:10:5, and an appropriate amount of N-methylpyrrolidone solvent ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wire diameter | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com